Discussion
Several laser therapy options for the treatment of basal cell carcinoma have been described including carbon dioxide laser (Adams and Price 1979, Wheeland et al 1987, Fairhurst et al 1992, Grobbelaar et al 1997, Krunic et al 1998, Humpreys et al 1998, Horlock et al 2000, Nouri et al 2002, Campolmi et al 2002, Robinson et al 2003), erbium:YAG laser (Smuckler et al 2008), Nd:YAG laser (El-Tonsy et al 2004, Moskalik et al 2009), pulsed dye laser (Allison et al 2003, Campolmi et al 2008, Shah et al 2009, Konnikov et al 2011), alexandrite laser (Ibrahimi et al 2011) and photodynamic therapy (Star et al 2006, Chapas et al 2006, Brathen et al 2007).
With non-ablative lasers as pulsed dye laser (Allison et al 2003, Campolmi et al 2008, Shah et al 2009, Konnikov et al 2011), Nd:YAG laser (El-Tonsy et al 2004, Moskalik et al 2009) and alexandrite laser (Ibrahimi et al 2011), the treatment of BCC is based on the angiogenic component presented in this tumour. Usually, several sessions of treatment should be performed in order to eliminate the lesion. The elimination of deep lesions was not achieved by these techniques due to the limited depth of penetration of these lasers.
With ablative lasers as carbon dioxide laser (Adams and Price 1979, Wheeland et al 1987, Fairhurst et al 1992, Grobbelaar et al 1997, Krunic et al 1998, Humpreys et al 1998, Horlock et al 2000, Nouri et al 2002, Campolmi et al 2002, Robinson et al 2003) and erbium:YAG laser (Smuckler et al 2008), the angiogenic component being a guide to the surgeon that indicates the elimination of the tumour, the lesion can be vaporized as deep as possible with the increase of the passes number. The ability to vaporize of carbon dioxide laser is higher than erbium:YAG laser and the majority of studies concerning the treatment of BCC with ablative lasers was performed with CO2 laser.
CO2 laser treatments treatments might be used in the treatment of superficial or nodular BCC. In morpheiphorm or infiltrative cases, these destructive techniques were not indicated because they do not allow histopathological study of the lesion margins. It is possible save a minimal amount of tissue previous to laser vaporization for histopathological confirmation of the diagnosis, but the absence of tumour infiltration in the lesion margins cannot be defined.
Carbon dioxide laser treatment of BCC have been largely described several years ago but few studies have been performed and they showed very different results (Adams and Price 1979, Wheeland et al 1987, Humpreys et al 1998, Horlock et al 2000, Campolmi et al 2002).
Adams and Price (1979) reported the use of the carbon dioxide laser to treat BCC in 1979 using a continuous output laser. They treated 25 BCCs with a single non-overlapping pass and performed postoperative histologic analyses with persistence of the tumour on treated skin in 50% of biopsies.
Wheeland et al (1987) treated 370 superficial BCCs with one to three passes of a continuous mode CO2 laser in conjunction with curettage between passes. They followed patients clinically for a period of 6 to 65 months (mean 19.9 months) and found no evidence of recurrence in the treated tumours. Although hypertrophic scarring occurred in 5% of patients, the authors emphasize the advantages of laser treatment, including minimal postoperative pain, rapid healing and superior cosmetic results.
Horlock et al (2000) treated 21 superficial, 28 nodular and 2 infiltrative basal cell carcinomas with multiple passes until clinical resolution was obtained and subsequently treated with two additional passes. Nodular tumours less than 10 mm diameter were completely ablated if they were vaporize to a depth of the lower dermis or deeper, whereas large nodular tumors greater than 10 mm had a high incomplete ablation rate. The study did not include long-term clinical evaluations of the patients.
Iyer S et al (2004) performed a retrospective review of patients with both nodular and superficial BCCs treated with the UltraPulse CO2 laser. Of the 61 tumours treated, clinical recurrence was observed in two cases (3.2%). Adverse effects included significant hypertrophic scarring in one patient and hypopigmentation in the other.
Destruction of superficial and nodular BCC may be accomplished successfully and safely with the UltraPulse CO2 laser with a cure rate of 97% (Jung et al 2011).
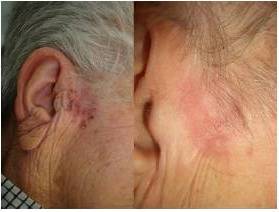
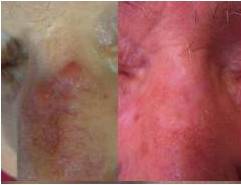
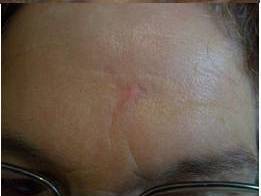
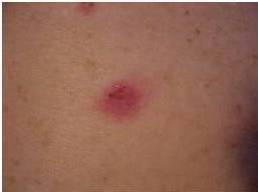
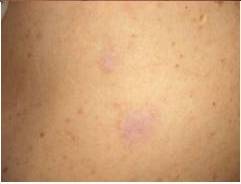
The main secondary effects after CO2 laser treatment were persistent erythema, hypopigmented areas and textural alterations. Persistent erythema appeared in all cases when important areas were treated. It is a vascular temporal reaction induced by the healing tissue. Hypopigmented areas resulted from the elimination of the melanocytes during laser treatment. In the healing process, the pigmentation of the treated area was recovered by the peripheral or follicular melanocytes, but in some cases it was not possible and this secondary effect was permanent. Textural alterations appeared when a deep vaporization was performed and medium or deep dermis was destroyed.
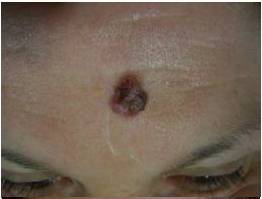
Superficial and nodular BCC are usually the most adequate types to be treated with carbon dioxide laser vaporization, with excellent results. Sclerodermiform or morpheiphorm variants of basal cell carcinoma not been due treated with this modality.
Ultra-Pulse CO2 ablation confers the following advantages in:
- In patients with numerous and large lesions, CO2 laser had minimal postoperative morbidity and shorter postoperative healing time,
- The highly precise and confined tissue damage and therefore potentially better cosmesis,
- The treatment therefore offers a virtually bloodless intraoperative field,
- The postoperative evolution by a minimal postoperative pain,
- The follow-up it is easy to repeat the treatment if it is necessary.
The main limitations of carbon dioxide laser vaporization of basal cell carcinomas are the following:
- The disponibility and the price of the treatment,
- The absence of histopathological control of the treatment,
- The experience of the surgeon who performs the treatment,
- The presence of secondary effects, essentially scarring and hypopigmentation. These secondary effects are more frequent in nodular cases of basal cell carcinoma because of the fact that more deep treatment is necessary to clear the tumour.
At this moment the evidence level is: Carbon dioxide laser ablation may be effective in the treatment of low-risk BCC. (Strength of recommendation C, quality of evidence III). Nevertheless, the results show that carbon dioxide laser might be an effective treatment with low recurrences. More studies are necessary in order to increase the evidence level.
Conclusion
“97% long-term cure rate in patients with basal carcinoma treated with the UltraPulse CO2 laser can be achieved. Although surgical excision remains the treatment of choice for basal cell carcinoma, CO2 laser ablation offers many advantages in specific situations as previously discussed.”
(adsbygoogle = window.adsbygoogle || []).push({});
References
Adams, E. L. & Price, N. M. (1979). ‘Treatment of Basal Cell Carcinoma with a Carbon Dioxide Laser,’J Dermatol Surg Oncol; 5: 803–6.
Allison, K. P., Kiernan, M. N., Waters, R. A. & Clement, R. M. (2003). “Pulsed Dye Laser Treatment of Superficial Basal Cell Carcinoma: Realistic or Not?,” Lasers in Medical Science; 18: 125-6.
Publisher – Google Scholar
Al-Othman, M. O. F., Mendenhall, W. M. & Amdurm, R. J. (2001). “Radiotherapy Alone for Clinical T4 Skin Carcinoma of the Head and Neck with Surgery Reserved for Salvage,” American Journal of Otolaryngology (2001); 22: 387–90.
Publisher – Google Scholar
Barlow, J. O., Zalla, M. J., Kyle, A., et al (2006). “Treatment of Basal Cell Carcinoma with Curettage Alone,” Journal of the American Academy of Dermatology; 54: 1039–45.
Publisher – Google Scholar
Bath-Hextall, F., Perkins, W., Bong, J. & Williams, H. C. (2007). “Interventions for Basal Cell Carcinoma of the Skin,” Cochrane Database of Systematic Reviews; 1:CD003412.
Publisher – Google Scholar
Bieley, H. C., Kirsner, R. S., Reyes, B. & Garland, L. D. (1992). “The Use of Mohs Micrographic Surgery for Determination of Residual Tumor in Incompletely Excised Basal Cell Carcinoma,” Journal of the American Academy of Dermatology; 26: 754–6.
Publisher – Google Scholar
Braathen, L. R., Szeimies, R.- M., Basset-Seguin, N., et al (2007). “Guidelines on the Use of Photodynamic Therapy for Nonmelanoma Skin Cancer. An International Consensus,” Journal of the American Academy of Dermatology; 56:125–43.
Publisher – Google Scholar
Braun, R. P., Klumb, F., Bondon, D., et al (2005). “Three-Dimensional Reconstruction of Basal Cell Carcinomas,” Dermatologic Surgery; 31: 562–6.
Publisher – Google Scholar
Campolmi, P., Brazzini, B., Urso, C., Ghersetich, I., Mavilia, L., Hercogova, J. & Lotti, T. (2002). “Superpulsed CO2 Laser Treatment of Basal Cell Carcinoma with Intraoperatory Histopathologic and Cytologic Examination,” Dermatologic Surgery; 28: 909-11.
Publisher – Google Scholar
Campolmi, P., Troiano, M., Bonan, P., Cannarozzo, G. & Lotti, T. (2008). “Vascular Based Non Conventional Dye Laser Treatment for Basal Cell Carcinoma,” Dermatologic Therapy; 21: 402-5.
Publisher – Google Scholar
Chapas, A. M. & Gilchrest, B. A. (2006). “Broad Area Photodynamic Therapy for Treatment of Multiple Basal Cell Carcinomas in a Patient with Nevoid Basal Cell Carcinoma Syndrome,” Journal of drugs in dermatology; 5(2 Suppl): 3-5.
Publisher – Google Scholar
El-Tonsy. M. H., El-Domyati, M. M., El-Sawy, A. E., El-Din, W. H., Anbar, Tel-D. & Raouf, H. A. (2004). “Continuous-Wave Nd:Yag Laser Hyperthermia: A Successful Modality in Treatment of Basal Cell Carcinoma,” Dermatology Online Journal; 15; 10(2): 3.
Publisher – Google Scholar
Fairhurst, M. V., Roenigk, R. K. & Brodland, D. G. (1992). “Carbon Dioxide Laser Surgery for Skin Disease,” Mayo Clinic Proceedings; 67: 49–58.
Publisher – Google Scholar
Felder, S., Rabinovitz, H. & Oliviero, M. & Kopf, A. (2006). “Dermoscopic Differentiation of a Superficial Basal Cell Carcinoma and Squamous Cell Carcinoma in Situ,” Dermatologic Surgery; 32: 423–5.
Publisher – Google Scholar
Greenway, N. T., Cornell, R. C., Tanner, D. J., et al (1986). “Treatment of Basal Cell Carcinoma with Intralesional Interferón,” Journal of the American Academy of Dermatology; 15: 437-43.
Publisher – Google Scholar
Grobbelaar, A. O., Horlock, N. & Gault, D. T. (1997). “Gorlin’s Syndrome: The Role Carbon Dioxide Laser in Patient Management,” Annals of Plastic Surgery; 39: 366–73.
Publisher – Google Scholar
Goldman, L., Rockwell, R. J. Jr, Meyer, R. & Otten, R. (1968). “Investigative Studies with the Laser in the Treatment of Basal Cell Epitheliomas,” Southern Medical Journal; 61: 735-42.
Publisher – Google Scholar
Goldman, L. & Wilson, R. G. (1964). “Treatment of Basal Cell Epithelioma by Laser Radiation,” JAMA; 189: 773-5.
Publisher – Google Scholar
Horlock, N., Grobbelaar, A. O. & Gault, D. T. (2000). “Can the Carbon Dioxide Laser Completely Ablate Basal Cell Carcinomas? A Histologic Study,” British Journal of Plastic Surgery; 53: 286–93.
Publisher – Google Scholar
Humpreys, T. R., Malhotra, R., Scharf, M. J., Marcus, S. M., Starkus, L. & Calegari, K. (1998). “Treatment of Superficial Basal Cell Carcinoma and Squamous Cell Carcinoma in Situ with a High-Energy Pulsed Carbon Dioxide Laser,” Archives of Dermatology; 134: 1247-52.
Publisher – Google Scholar
Ibrahimi, O. A., Sakamoto, F. H., Tannous, Z. & Anderson, R. R. (2011). “755 nm Alexandrite Laser for the Reduction of Tumor Burden in Basal Cell Nevus Syndrome,” Lasers in Surgery and Medicine; 43: 68-71.
Publisher – Google Scholar
Iyer, S., Bowes, L., Kricorian, G., Friedli, A. & Fitzpatrick, R. E. (2004). “Treatment of Basal Cell Carcinoma with the Pulsed Carbon Dioxide Laser: A Retrospective Analysis,” Dermatologic Surgery; 30: 1214–1218.
Publisher – Google Scholar
Jung, D.- S., Cho, H.- H., Ko, H.- C., Bae, Y.- C., Oh, C.- K., Kim, M.- B. & Kwon, K.- S. (2011). “Recurrent Basal Cell Carcinoma Following Ablative Laser Procedures,” Journal of the American Academy of Dermatology; 64: 723-9.
Publisher – Google Scholar
Kagy, M. K. & Amonette, R. (2000). “The Use of Imiquimod 5% Cream for the Treatment of Superficial Basal Cell Carcinomas in a Basal Cell Nevus Syndrome Patient,” Dermatologic Surgery; 26: 577-579.
Publisher – Google Scholar
Kokoszka, A. & Scheinfeld, N. (2003). “Evidence-Based Review of the Use of Cryosurgery in Treatment of Basal Cell Carcinoma,” Dermatologic Surgery; 29: 566–71.
Publisher – Google Scholar
Konnikov, N., Avram, M., Jarell, A. & Tannous, Z. (2011). “Pulsed Dye Laser as a Novel Non-Surgical Treatment for Basal Cell Carcinomas: Response and Follow up 12-21 Months after Treatment,” Lasers in Surgery and Medicine; 43: 72-8.
Publisher – Google Scholar
Krunic, A. L., Viehman, G. E., Madani, S. & Clark, R. E. (1998). “Microscopically Controlled Surgical Excision Combined with Ultrapulse CO2 Vaporization in the Management of a Patient with the Nevoid Basal Cell Carcinoma,” The Journal of Dermatology; 25: 10–2.
Publisher – Google Scholar
Lo, J. S., Snow, S. N., Reizner, G. T., et al (1991). “Metastatic Basal Cell Carcinoma: Report of Twelve Cases with a Review of the Literature,” Journal of the American Academy of Dermatology; 24: 715–19.
Publisher – Google Scholar
Mallon, E. & Dawber, R. (1966). “Cryosurgery in the Treatment of Basal Cell Carcinoma. Assessment of One and Two Freeze-Thaw Cycle Schedules,” Dermatology Surgery; 22: 854–8.
Publisher – Google Scholar
McGuff, P. E. (1966). “Laser Radiation for Basal Cell Carcinoma,” Dermatologica; 133: 379-83.
Publisher – Google Scholar
Moskalik, K., Kozlov, A., Demin, E. & Boiko, E. (2009). “The Efficacy of Facial Skin Cancer Treatment with High-Energy Pulsed Neodymium and Nd: YAG Lasers,” Photomedicine and Laser Surgery; 27: 345-9.
Publisher – Google Scholar
Nouri, K., Chang, A., Trent, J. T. & Jimenez, G. P. (2002). “Ultrapulse CO2 Used for the Successful Treatment of Basal Cell Carcinomas Found in Patients with Basal Cell Nevus Syndrome,” Dermatologic Surgery; 28:287–90.
Publisher – Google Scholar
Robinson, J. K., Hernandez, C., Anderson, E. R. & Nickoloff, B. (2003). “Topical and Light-Based Treatments for Basal Cell Carcinoma,” Seminars in Cutaneous Medicine and Surgery; 22: 171-6.
Publisher – Google Scholar
Rowe, D. E., Carroll, R. J. & Day, C. L. (1989). “Mohs Surgery is the Treatment of Choice for Recurrent (Previously Treated) Basal Cell Carcinoma,” The Journal of Dermatologic Surgery and Oncology; 15: 424–31.
Publisher – Google Scholar
Schulze, H. J., Cribier, B., Requena, L., et al (2005). “Imiquimod 5% Cream for the Treatment of Superficial Basal Cell Carcinoma: Results from a Randomized Vehicle-Controlled Phase III Study in Europe,” British Journal of Dermatology; 152: 939–47.
Publisher – Google Scholar
Shah, S. M., Konnikov, N., Duncan, L. M. & Tannous, Z. S. (2009). “The Effect of 595 nm Pulsed Dye Laser on Superficial and Nodular Basal Cell Carcinomas,” Lasers in Surgery and Medicine; 41: 417-22.
Publisher – Google Scholar
Smucler, R. & Vlk, M. (2008). “Combination of Er: YAG Laser and Photodynamic Therapy in the Treatment of Nodular Basal Cell Carcinoma,” Lasers in Surgery and Medicine; 40: 153-8.
Publisher – Google Scholar
Spiller, W. F. & Spiller, R. F. (1984). “Treatment of Basal Cell Epitheliomas by Curretage and Electrodessication,” Journal of the American Academy of Dermatology; 11: 808-814.
Publisher – Google Scholar
Star, W. M., Van’t Veen, A. J., Robinson, D. J., Munte, K., de Haas, E. R. & Sterenborg, H. J. (2006). “Topical 5-Aminolevulinic Acid Mediated Photodynamic Therapy of Superficial Basal Cell Carcinoma Using Two Light Fractions with a Two-Hour Interval: Long-Term Follow-up,” Acta Dermato Venereologica; 86: 412-7.
Publisher – Google Scholar
Telfer, N. R., Colver, G. B. & Morton, C. A. (2008). “Guidelines for the Treatment of Basal Cell Carcinoma,” British Journal of Dermatology; 159: 35-48.
Publisher – Google Scholar
Van der Geer, S., Ostertag, J. U. & Krekels, G. A. (2009). “Treatment of Basal Cell Carcinomas in Patients with Nevoid Basal Cell Carcinoma Syndrome,” Journal of the European Academy of Dermatology and Venereology; 23: 308-13.
Publisher – Google Scholar
Walker, P. & Hill, D. (2006). “Surgical Treatment of Basal Cell Carcinomas Using Standard Postoperative Histological Assessment,” Australasian Journal of Dermatology; 47: 1–12.
Publisher – Google Scholar
Wheeland, R. G., Bailin, P. L., Ratz, J. L. & Roenigk, R. K. (1987). “Carbon Dioxide Laser Vaporization and Curettage in the Treatment of Large or Multiple Superficial Basal Cell Carcinomas,” TheJournal Dermatologic Surgery and Oncology; 13: 119–25.
Publisher – Google Scholar