Introduction
The quality of life of breast cancer survivors beyond the acute phase of treatment have been the subject of several clinical studies (Perry et al 2007, Arndt et al 2008, Ganz 2008). Although the majority of cancer survivors previously treated for early-stage breast cancer are consistently reporting improvement in the quality of life (Tomich et al 2005, Bardwell et al 2004), adverse psychosocial impact of breast cancer may affect some patients long after the treatment have been terminated: Distress arrising from cancer diagnosis and treatment, body image disruption, loss of attractiveness and fear of the disease recurrence may negatively influence the patient’s cognitive and emotional functioning, marital, sexual, family, and social relationships and the health-related quality of life (Panagopoulou et al 2002, Hewitt et al. 2004 Anderson et al 2012, Giese-Davis et al 2012). Kornblith and coworkers (2007) measuring adjustment, physical functioning and treatment-related physical problems reported worse psychosocial adjustment of younger breast cancer survivors one year after treatment.
The increasing use of screening ultrasonography and mammography have lead to improvement of early stage breast cancer detection, that may be treated by breast conserving surgery followed by adjuvant radiotherapy.. Recent findings suggest that breast conserving surgery (lumpectomy, BCS) and modified radical mastectomy (MRM) appear to be equivalent in terms of patient”s survival time (Kenny et al 2000). Breast conserving surgery is now regarded as a standard modality for the treatment of early-stage breast cancer. Regardless the extend of the surgical intervention, both modalities were associated with adverse effect onto quality of life of breast cancer survivors (Ohsumi et al 2007). Despite the efficacy of non-invasive treatment with breast conservation, also patients treated with lumpectomy face a risk of cancer recurrence. In both breast cancer patients groups, the breast cancer risk status is leading to the development of psychosocial morbidity reflected by anxiety, fears, concerns, uncertainty, emotional disruption, loss of personal functioning, nervousness, physical disruptions, fatigue and social isolation (Moyer 1997, Klassen et al 2009). The emotional burden of these patients is frequently considered irrelevant and their psychosocial support needs are neglected despite the fact that psychosocial parameters associated with breast cancer and its treatment are dysfunctioning social network, minimization, denial and coping and may predict patient‘s survival ( Petigrew et al 2002, Kroenke et al 2006, Phillips et al 2008).
Although breast conserving surgery has been shown to confer psychosocial benefits in breast cancer survivors (Kornblith et al 2007 ), negative psychological outcomes can develop long after the surgical intervention in the course of regular follow-up: In expectation of the follow-up clinical results, intrusive thoughts about future, uncertainty about the health outcome and repeated anxiety periods are resulting in the decline of the quality of life due to repeated psychosocial distress (Ganz et al 2002). Since the psychosocial burden related to breast cancer treatment and survival of Slovak patients was previously not extensively studied, the aim of this survey was to assess age-related changes in quality of life and psychosocial parameters among breast cancer patients surviving three years after BCS.
Material and Methods
Patients
140 patients surviving after breast cancer surgery were recruited during their regular post treatment follow-up at the Ist. Clinic of Oncoly, Faculty of Medicine, Comenius University, Bratislava. The sample consisted of 89 women (aged 35 – 73 years) treated for early-stage breast cancer (Tis-T2a) by BCS, and 51 women (aged 39 – 76 years) with advanced breast cancer (T2a-T3) treated by MRM. Only relapse-free patients without serious comorbidities surviving more than three years after surgery and responding the questionnaires at desired survival periods of time were involved. In an attempt to exclude direct clinical consequences of treatment onto psychosocial status and quality of life, patients were enrolled into the study one year (+- 3, 4 months) after termination of the treatment. Three years later (+- 4, 5 months) 6 BCS-treated patients were not presented themselves for assessment; one patient relapsed and was withdrawn from the survey. In the MRM-treated group, 5 patients had moved to an unknown address, 2 patients had recently died. The health-related quality of life, psychosocial morbidity and social status of breast cancer survivors was investigated in a follow-up study in both study arms. 126 patients (82 BCS-treated, 44 MRM-treated) completed the study questionnaires and were enrolled into the evaluation.
All MRM-treated patients received post-surgical radiotherapy, while only 21 (25, 9%) BCS-treated patients (mainly T2a) were treated with both modalities.
BCS-treated patients were divided into five age groups and the statistically significant difference in the subscale data of psychosocial functioning was assessed by means of the non-parametric Kruskal-Wallis, van der Waerden, and Siegel-Tukey tests (Eviews5 softwer). Kruskal-Wallis is a rank-based procedure of location equality for three or more groups used in behavioral and social science research. Van der Waerden and Siegel-Tukey test converts the results from a standard Kruskal-Wallis variance analysis to quantiles of normal distribution (Jang et al 2009).
Table 1: Time-Related Quality of Life among Breast Cancer Patients Survivors One and Three Years after BCS Vers. MRM (QLQ C30.3 Questionnaire, Item 29)
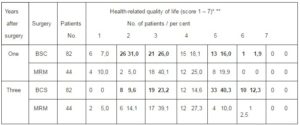
* 1 – very poor – 7 – excellent
** BSC mean score: year 1 = 3,2 year 2 = 4,8
Quality of Life and Psychosocial Status Assessment
The European Organization of Research and Treatment of Cancer questionnaire EORTC QLQ-C30.3 (Aaronson et al 1993) is a 30-item questionnaire comprised of nine domains: physical symptoms, functioning, cognitive, emotional and social status, fatigue, pain, nausea and vomiting. Ten point difference on a 0 – 100 scale of the EORTC QLQ-C30.3 suite has been considered as treshold for clinically important difference.
The EORTC QLQ B-23 module (Sprangers et al 1996) is a 23-item breast cancer specific questionnaire composed of five domains: body image, sexuality, and three breast specific physical symptoms.
The Hospital Anxiety and Depression Scale (Zigmond and Snaith 1983) is a 14-item questionnaire oriented toward assessment of syndromes related to anxio-depressive disorders. The validity and specificity of the HADS questionnaire was proved for hospitalized as well as of outdoor cancer patients.
The EORTC QLQ questionnaires and the HADS questionnaire were officially translated into the Slovak language and were available upon request.
Scoring of the QLQ questionnaires was performed according to the EORTC scoring manual (Fayers et al 2001). Incompletely fulfilled questionnaires were discarded from the calculations. The items were adjusted to a 1 – 100 linear points scale and were statistically evaluated. The HADS scoring was calculated according to a scheme recommended by Phillips and co-workers (2008) whereby patient’s responses scored 1 – 7 were considered as normal, 8 -10 borderlines, and 11 – 21 of clinical levels of both, anxiety and depression subscale.
Results
Time-Related Difference of Health-Related Quality of Life in Breast Cancer Survivors after BCS versus MRM
The health-related quality of life of breast cancer survivors one and three years after BCS versus MRM is presented in Table.1.: Using the 1-7 linear quality of life scale (very poor – excellent (QLQ-C30.3, item 29), the survey showed clear differences in scoring among groups of patients surviving from breast cancer after BCS or MRM one year and three years after surgery: Regardless of age, breast cancer patients surviving after MRM scored nearly uniformly low (score 2–3, „poor – rather poor“) and three years after surgery. The slight quality of life improvement in this group at the three-year survival (44, 9 vers. 37, 3%) have shown to be not significant. Identical low quality of life scoring has been registered in patients surviving one year after BCS. Three years after treatment, BSC – treated patients’ scores are considerably different: 52% patients surviving three years after BCS scored between 5 – 6 (good – very good) providing improvement in their quality of life. Although majority of BCS survivors being three years after surgery scored their quality of life relatively high, at least 33% survivors from this group of patients considered their health-related quality of life as poor or not good. (quality of life score 2 – 3). Moreover, none of the responders of this study arm classified their quality of life as excellent.
Age-Related Differences in the Quality of Life among BCS-Treated Survivors One and Three Years after Surgery
Eighty two BCS-treated breast cancer survivors were divided into five age groups (29-40, 41-50, 51-60, 61-70, over 70 years) and the health related quality of life was assessed in each group separately one and three years after surgery and was statistically evaluated. Furthermore, statistical significance in the quality of life of patients under age 50 (28 patients) and over age 50 (54 patients) was analysed. The results summarised in Table 2 have shown that:
a) One year after BCS there is no statistically significant difference in the quality of life score between patient age groups (P=0,1051 <0,05).
b) Statistically significant decrease in the quality of life score from the first to third year after surgery has been observed in patients groups under 50 years of age (mean value 5,25 vers. 4,31 p=0,0016<0,05), while in patients over 50 years of age increase of quality of life scores three years after surgery revealed slight but statistically significant improvement (mean value 5,14 vers. 5,69 p=0,0015<0,05). The comparative analysis of the quality of life in patients 50 three years after surgery using the Siegel-Tukey test confirmed previous observation (mean value 4, 79 vers. 5, 47 p=1, 9603<0, 05). Further analysis has shown that the mean quality of life scores during the whole assessment time was significantly lover in younger age group in comparison with patients over 50 years of age. (p=0, 0014<0, 05).
Table 2. Difference of Age-Related Quality of Life Score among Breast Cancer Patients Surviving One and Three Years after Breast-Conserving Surgery (QLQ C30.3 Item 29)
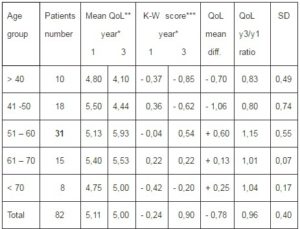
*Years of survival after breast-conserving surgery
**Mean (1 – 7) quality of life score
***Kruskal-Wallis test for equality between series: score 0,1051, p<0,05 after one year
of survival, score 0,0000 after three years survival.
Breast Cancer-Specific Psychosocial Distress Development in Breast Cancer Survivors after BCS Treatment
Table 3 presents the subscale scores of psychosocial functioning for breast cancer patients surviving one and three years after BCS versus MRM as assessed by the QLQ-30.3 questionnaire.
The Likert scale scores (1 – 4) were linearly transformed to 1 – 100 scale by means of the EORTC QLQ Manual schedule (Zigmond and Snaith, 1983).
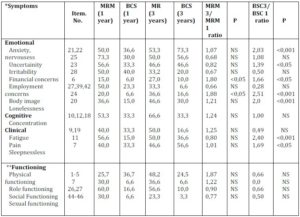
In the follow-up study, breast cancer patients surviving one and subsequently three years after MRM did not considerably differ in their emotional dysfunction scores. Except for limited improvement in financial and employment scores, sexual functioning, and to some extent body image perception, these patients reported persistently higher scores of psychosocial distress symptomatology (anxiety 50,0 – 53,3 uncertainty 73,3 – 50,0, irritability 56,6 – 46,6), fatigue (53,3 – 66,6), pain (40,0 – 50,0), and persistence of concerns about the future (30,0 – 60,6). On the contrary, psychosocial burden scores in patients after BCS showed different survival-dependent dynamics: Although these patients scored considerably lower one year after BCS, at three years post-treatment the BCS – treated breast cancer survivors reported significantly higher levels of anxiety (ratio 2, 03 p<0,001), irritability (ratio 1, 39 p<0,005), lineliness (ratio 2, 51 p<0,001) and future perspective (ratio 2, 72 p<0,001).
The adverse effect of clinical cancer symptoms including side effect of treatment on the psychosocial status of breast cancer patients has been well documented. Our data of clinical symptomatology scores (arm symptoms, breast symptoms, pain) of breast cancer patients surviving three years after BCS have shown to be rather low and apparently without any adverse effect on the quality of life and psychosocial profile of BCS-treated patients.
Anxiety versus Depression Ratio Related to the Psychosocial Distress Symptomatology of Breast Cancer Patients Surviving One and Three Years after BCS
The observed time-related dynamics of psychosocial morbidity developing after breast-conserving surgery indicated that these patients continue to experience adverse psychosocial disorders that may increase with the survival time. The HADS scoring presented in Table 4 has shown that there was a remarkable difference in the time-related scores on the anxiety subscale. While the mean +- SD sample score one year after BCS was 8,2 (SD 0,34), the very same sample score three years after BCS showed significant increase with mean +- 12,7 (SD 0,75) score, whereas no significant score difference in the depression subscale in relation to survival time have been shown. These results brought additional evidence indicating that higher anxiety scores were noted in patients regardless of the post-operation survival time, and anxiety scores showed increasing course with the function of survival time.
Table 4. Anxiety and Depression Symptomatology in Breast Cancer Survivors One and Three Years after Breast Conserving Surgery As Assessed by the Hospital Anxiety and Depression Scale Qestionaire (HADS)
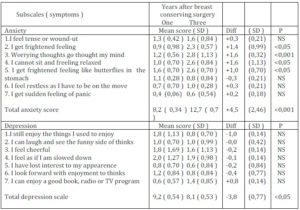
Anxiety scale (0 – 3: never – frequently)
Depression scale (0 – 3: most of the time – rarely)
Discussion
Few data are available on the long-term follow-up studies analysing psychosocial distress among breast cancer survivors treated by breast cancer surgery. Arndt and coworkers (2005) demonstrated that the deficits in role, emotional, cognitive, and social functioning persist years after MRM and affect predominantly younger patients.. Patients three years after MRM showed only modest improvements with respect to emotional functioning, future perspective, and breast symptoms, whereas persistent psychosocial deteriorations were observed during the whole observation period. Mistakidou and coworkers (2005) in comparing the BSC-treated breast cancer patients’ responses to the EORTC QLQ-C30.3 questionnaire with responses to HADS questionnaire have showed that the association between decreased quality of life and increased psychosocial sequelae in breast cancer survivors was based on emotional functioning distortion due to repeated episodes of anxiety and depression. Despite these observations, qualitative and quantitative changes occurring over a protracted period of time in patients after breast conserving therapy are not well understood. The results of our survey confirm previously published analyses (Thewers et al 2004, Burgess et al 2005) indicating that there is a considerably high proportion of predominantly younger long-term breast cancer survivors previously treated by BCS who continue to experience adverse psychosocial disorders that may increase in their intensity over the survival time (Wallberg 2011) Moreover, this increase may be attributed to increased frequency of episodes of anxiety, nervousness, irritability and uncertainty due to the fear of tumor recurrence. As presented by others, fear of cancer recurrence is dominating over items like sexual function disruption or body image perception (Engel et al 2004, Lehto et al 2006).
It has been well documented that the majority of patients treated for early breast cancer are suffering from anxiety and depression mainly in the first year after diagnosis, and the prevalence of anxiodepression syndrome has been shown to have a falling tendency of about 15% of cases each following year (Ganz et al 2008). However, substantial proportion of surviving patients is experiencing consistent psychosocial burden years after cancer treatment (Arndt et al 2008). Cohen and coworkers (2000) have shown that women of younger age, who had BCS, experienced significantly greater psychosocial distress and marginally worse quality of life 40 months after surgery. The present study clearly demonstrates that the psychosocial distress experienced by some young Slovak women treated for early-stage breast cancer by BCS is reflected by a whole range of anxiety symptoms even three years after surgery and that the anxiety may increase within the post-treatment time. The data brought additional evidence indicating that cancer-related anxiety syndrome has not only a negative impact onto quality of life but it is harming the role, family and social functioning of BCS-treated breast cancer survivors.
The interpretation of our results has some limitations. The persistence of emotional distress in younger patients surviving in long-term remission after BCS may be a result of a variety of reasons. The symptoms of anxiety among BCS-treated patients three years after surgery may be attributed to the fear of „bad news“from clinicians at each repeated regular check-up. Concerns about patient’s perception of their risk of recurrence may be influenced by insufficient doctor-patient communication. Without appropriate and more detailed explanation of the current status of the disease, and information concerning individual risk of cancer recurrence and prognosis, patients may suffer from uncertainty about their future. Doctors overloaded with clinical duties and managerial and economic pressure have limited time in the course of the ambulatory investigation to offer patients more detailed information about their disease. Furthermore, there is an urgent need to improve Slovak doctors’ communication skills: A basic training program of doctor-patient communication should be involved into undergraduate study program of medicine and the skills and knowledge should be deepened by specific training modules in postgraduate and specialisation studies. In respect to more precise evaluation of the achieved results, deeper analysis of the relationship to other variables like marital and social status, education, employment, spirituality etc. should be performed. Moreover, larger trials focused more precisely on the extent of age-related psychosocial burden of breast cancer survivors treated by breast-conserving surgery and adjuvant radiochemotherapy are needed to further explore these results.
Conclusion
Although anxiety and depression are common in breast cancer survivors and their negative impact on treatment outcomes and coping abilities is well documented, they are frequently not taken into consideration and left untreated. In Central Europe including Slovakia the role of well educated psychosocial oncology workers positioned within the healthcare providers team (Davis 2004) is completely ignored. Therefore, one of the goals of the present study was to highlight the urgency to improve doctor-patient communication skills and to pay more attention to controlled professional follow-up psychosocial care for all cancer patients including breast cancer survivors after breast-conserving surgery who have survived for more than three years even without clinical symptoms of the disease. The increase of outpatient psychosocial care should be emphasized.
Acknowledgements
The authors wish to express their gratitude to Ms. Zuzana Krajcovicova, CERGE-EI, Prague, Czech Republic, for kind statistical evaluation of our results.
(adsbygoogle = window.adsbygoogle || []).push({});
References
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J. Filiberti, A., Flechtner, H., Fleishman, S. B., de Haes, J. C. J. M., Kaasa, S., Klee, K., Osoba, D., Razavi, D., Rofe, P. B., Schraub, S., Sneeuw, K., Sullivan, M. & Takeda, F. (1993). “The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality of Life Instrument for Use in International Clinical Trials in Oncology,“ Journal of the National Cancer Institute 85: 365-376
Publisher – Google Scholar
Anderson, J. H., Ganz, P. A., Bower, J. E. & Stanton, A. L. (2012). “Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors,” Journal of the National Cancer Institute 104(5): 386 – 405
Publisher – Google Scholar
Arndt, V., Merx, H., Stegmayer, C., Ziegler, H. & Brenner, H. (2005). “Persistence of Restrictions in Quality of Life from the First to the Third Year after Diagnosis in Women with Breast Cancer,“ Journal of Clinical Oncology. 23: 4945-4953
Publisher – Google Scholar
Arndt, V., Stegmaier, C., Ziegler, H. & Brenner, H. (2008). “Quality of Life over 5 Years in Women with Breast Cancer after Breast-Conserving Therapy versus Mastectomy: A Population-Based Study,” Journal of Cancer Research and Clinical Oncology 134 (12): 1311 – 1318
Publisher – Google Scholar
Bardwell, W. A., Major, J. M., Rock, C. L., Newman, v. A., Thomson, C. A., Colton, J. A., Dimsdale, J. E. & Pierce, J. P. (2004). “Health-Related Quality of Life in Women Previously Treated for Early-Stage Breast Cancer,” Psycho-Oncology 13: 595 – 604
Publisher – Google Scholar
Burgess, C., Cornelius, V., Love, S., Graham, J., Richards, M. & Ramirez, A. (2005). “Depression and Anxiety in Women with Early Breast Cancer: Five Years Observation Cohort Study,” British Medical Journal 330: 702 – 714
Publisher – Google Scholar
Cohen, L., Hack, T. F., deMoor, C., Katz, J. & Gross, P. E. (2000). “The Effects of Type of Surgery and Time on the Psychosocial Adjustment in Women after Breast Cancer Treatment,” Annals of Surgical Oncolology 7: 427 – 434
Publisher – Google Scholar
Davis, C. (2004). “Psychosocial Needs of Women with Breast Cancer: How Can Social Workers Make a Difference?,” Health and Social Work 29: 330 – 334
Publisher – Google Scholar
Engel, J., Kerr, J., Schlessinger-Raab, A., Sauer, H. & Hölzel, D. (2004). “Quality of Life Following Breast-Conserving Therapy or Mastectomy: Results of 5-Year Prospective Study,” Breast Journal 010: 223-231
Publisher – Google Scholar
Fayers, P. M., Aaronson, N. K., Bjordal, K., Groenfold, M. & Bortonley, A. (On behalf of the EORTC Quality of Life Group). (2001). ‘The EORTC Quality of Life Scoring Manual,’ 3rd ed., EORTC Press, Brussels, Belgium
Ganz, P. A., Desmond, K. A., Leedham, B., Rowland, J. H., Meyerowitz, B. E. & Belin, T. R. (2002). “Quality of Life in Long-Term, Disease Free Survivors of Breast Cancer: A Follow-Up Study,” Journal of the National Cancer Institute 94(1): 39 – 49
Publisher – Google Scholar
Ganz, P. A. (2008). “Psychological and Social Aspects of Breast Cancer,” Oncology 22(6): 246 – 254
Publisher – Google Scholar
Giese-Davis, J., Waller, A., Carlson, L. E., Groff, S., Zong, L., Neri, E., Bachor, S. M., Adamyk-Simpson, J., Rancourt, K. S., Dunlop, B. & Bultz, B. D. (2012). “Screening for Distress, the 6th Vital Sign: Common Problems in Cancer Outpatient over One Year in Usual Care: Associations with Marital Status, Sex, and Age,” BMC Cancer 12: 441 – 448
Publisher – Google Scholar
Hewitt, M., Herdman, R. & Holland, J. (2004). In the Book Meeting the Psychosocial Needs of Women with Breast Cancer, ‘The Effectiveness of Psychosocial Interventions for Women with Breast Cancer,’ p. 95 – 132, The National Academies Press, Wash., USA,
ISBN 0-309-O9129-2
Jang., Y, Chern, J. S. & Lin, K. C. (2009). “Validity of the Loewenstein Occupational Therapy Cognitive Assessment in People with Intellectual Disabilities,” American Journal of Ocupational Therapy 63: 414-422
Publisher – Google Scholar
Kenny, P., King, M. T., Sheil, A., Seymour, J., Hull, J., Langlsouds, A. & Boyages, J. (2000). “Early-Stage Breast Cancer, Costs and Quality of Life One Year after Treatment by Mastectomy or Conservative Surgery and Radiation Therapy,” The Breast 91: 1238 – 1246
Publisher – Google Scholar
Klassen, A. F., Pusic, A. L., Scott, A., Klok, J. & Canno, S. J. (2009). “Satisfaction and Quality of Life in Women Who Undergo Breast Surgery: A Qualitative Study,” BMC Women’s Health 9: 11 – 22
Publisher – Google Scholar
Kornblith, A. B., Powell, M., Regan, M. M., Bennett, S., Krasnek, C., Moy, B., Younger, J., Soodman, A., Berkowitz, R. & Winer, E. (2007). “Long-Term Psychosocial Adjustment of Older vers. Younger Survivors of Breast and Endometrial Cancer,” Psycho-Oncology 16: 895 – 903
Publisher – Google Scholar
Kroenke, C. H., Kubzansky, L. D., Schernhammer, E. S., Holmes, M. D. & Kawachi, I. (2006). ‘Social Network, Social Support, and Survival after Breast Cancer Diagnosis,’ Journal of Clinical Oncology 24: 1105, 1111
Lehto, U. S., Ojanen, M., Dyba, T., Aromaa, A. & Kellokumpu-Lehtinen, I. (2006). “Baseline Psychosocial Predictors of Survival in Locatised Breast Cancer,” British Journal of Cancer 94: 1245-1252
Publisher – Google Scholar
Moyer, A. (1997). “Psychosocial Outcomes of Breast-Conserving Surgery versus Mastectomy: A Meta-Analytic Review,“ Health Psychology 16 (5): 284 – 298
Publisher – Google Scholar
Mystakidou, K., Tsilika, E., Parpa, E., Katsouda, E., Galanos, A. & Vlahos, L. (2005). “Assessment of Anxiety and Depression in Advanced Cancer Patients and Their Relationship with Quality of Life,” Quality of Life Research 14: 1825 – 1833
Publisher – Google Scholar
Ohsumi, S., Shimozuma, K., Kuroi, K., Ono, M. & Imai, H. (2007). “Quality of Life of Breast Cancer Patients and Types of Surgery for Breast Cancer – Current Status and Unresolved Issues,” Breast Cancer 14(1): 66 – 73
Publisher – Google Scholar
Panagopoulou, E., Kersbergen, B. & Maes, C. (2002). “The Effects of Emotional (non)-Expression in (chronic) Disease: A Meta-Analytic Review,” Psychological. Health 17: 529-545
Publisher – Google Scholar
Perry, S., Kowalski, T. L. & Chang, C. H. (2007). “Quality of Life Assessment in Women with Breast Cancer: Benefits, Acceptability, and Utilization,” Health and Quality of Life Outcomes 5:24
Publisher – Google Scholar
Pettigrew, M., Bell, R. & Hunter, D. (2002). ‘Influence of Psychosocial Coping on Survival and Recurrence in People with Cancer Systemic Review,’ British Medical Journal 325: 1166-1069
Phillips, K. A., Osborne, R. H., Giles, G. G., Dite, G. S., Apicella, C., Hopper, J. L. & Milne, R. L. (2008). “Psychosocial Factors and Survival of Young Women with Breast Cancer: A Population-Based Prospective Cohort Study,” Journal of Clinical Oncology 26 (28): 4666-4671
Publisher – Google Scholar
Sprangers, M. A., Groenvold, M., Arraras, J. I., Franklin, J., teVerde, A. et al. (1996). “The European Organization for Research and Treatment of Cancer – Breast Cancer-specific Quality of life Questionnaire Module: First Results from a Three-Country Field Study,” Journal of Clinical Oncology 14: 2756 – 2768
Publisher – Google Scholar
Thewes, B., Buttow, P., Girgis, A. & Pendlebury, S. (2004). “The Psychosocial Needs of Breast Cancer Survivors: A Quabtitave Study of the Showed and Unique Needs of Younger versus Older Survivors,” Psychooncology 13(3): 177 – 189
Publisher – Google Scholar
Tomich, P. L., Helgeson, V. S. & Novak Vache, E. J. (2005). “Perceived Growth and Decline Following Breast Cancer: A Comparison to Age-Matched Controls 5 Years Later,” Psycho-Oncology 14: 1018 – 1029
Publisher – Google Scholar
Wallberg, B. (2011). “Breast Cancer Survivors. Information Needs, Attitudes toward Illness and Quality of Life,” Karolinska Institutet Publ., ISBN 978-91-7409-875-4
Zigmond, A. S. & Snaith, R. P. (1983). “The Hospital Anxiety and Depression Scale,” Acta Psychiatrica Scandinavica. 67: 361 – 370 24.
Publisher – Google Scholar






