Introduction
Water contaminants have become a hazard to human health and became environmental problem in India as well as in many parts of world. Heavy metal pollution of water is a major environment problem facing the modern world (Dushenkov et al., 1995). In addition, they are highly toxic for both higher organisms and microorganisms (Garbisu and Alkorta, 2001). Migration of chemicals through ground and surface water source in turn affects human health (Means et al., 1978). Moreover, it is gaining importance day by day due to its obvious impact on human health through the food chain (Prasad, 1997). The danger of heavy metals is aggravated by their almost infinite persistence in the environment because they cannot be destroyed biologically but are only transformed from oxidation state or organic complex to another.
Contaminants present in water source are microbial, inorganic and organic chemicals. In the last century,it was established that the introduction of the chlorinated water caused a large drop in the mortality from the infectious diseases. Chlorination produces many compounds containing chlorine and lower bromine. Some of which have been shown to be carcinogenic, mutagenic or teratogenic in animal studies (Abbas and Fisher, 1997).
Heavy metals also interact with RNA polymerases. Severe effects are expressed as such in RNA metal binding.RNA polymerase must bind site specifically to its RNA template, binds its nucleotide and primer substrates, and form a new phosphodiester bond in elongating the growing RNA.
Eukaryotic RNA polymerases I, II and III are involved in the synthesis of ribosomal, messenger and transfer RNAs, respectively. The RNA dependent RNA polymerases I (Falchuk et al., 1977), II (Falchuk et al., 1976) and III (Wandzilak and Benson, 1977) of the unicellular eukaryote Euglena gracilis have all been showed to be zinc metallo enzyme, each binding about 2 gram atoms of zinc.
The heavy metals bind with the DNA and cause the DNA damage, also the RNA polymerase are inactivated by heavy metals. These facts can reduce the rate of transcription and hence, the rate of the protein synthesis (Mahajan, 2006). Heavy metal, mercury affects DNA, contracts the chromatin and disturbs the protein synthesizing machinery of the cell resulting in to the decreased enzyme synthesis in hepatopancreas (Zambare and Mahajan 2001).
The role of RNA is to help protein synthesis in the cytoplasm, hence, depletion of RNA level also resulted decreased rate of protein synthesis (Rao et al., 1990). Similar decreased amount of RNA levels was observed by Choudhari et al., (1993) in Thiara lineata under different toxic stress. The cellular degradation, rapid histolysis and decreased rate of protein synthesis are the possible reasons.
Ester Saball et al., (2000) observed the total tissue m-RNA of liver and kidneys of control and HgCl2 treated rats. Tong Lu et al., (2001) observed that 10% genes, mostly related to cell cycle regulation, apoptosis, RNA damage response etc. were differentially expressed in the form of RNA and such abnormal RNA are vulnerable to RNA are attacked. Rao et al., (1998) studied the RNA levels in various tissues of freshwater crab Barytelphusa cunicularis when exposed to Fluoride. Thus, RNA levels in the tissues after exposure to heavy metals can be considered as the indices for stress.
Mercury poisoning shows the symptoms such as weakness, loss of appetite, loosening of teeth, insomnia, irritability, loss of memory, indigestion, diarrhea etc. Uptake of heavy metal by living organism causes the death. Mercury is recognized as toxic contaminants of our environment. These highly toxic heavy metals such as mercury enter into the body of living organism including man through non-vegetarian and vegetarian diet and drinking water and accumulate in the tissues. A main problem in toxic effect of heavy metals is that they are very difficult to remove from the body of animal, because they are usually bound to some legends. The heavy metals bind to the cell membrane. Therefore, they are very difficult to remove from cell membrane.
Chelators are particular substances that bind to heavy metals and speeds their elimination. The united states of public health service, in collaboration with the National Institutes of Health, organized a study of EDTA Chelation in 1981 and reported that EDTA Chelation therapy for arteriosclerosis should be considered experimental and without substantial incidence to support its clinical use. Most of the clinical reports, documenting appropriation of EDTA chelation for lead intoxication, originated in the early 1950s. According to the reports of American Heart Association, side effects of EDTA includes anemia, blood clotting, bone marrow damage, fever, insulin shock, irregular heartbeat, kidney damage, joint pain, difficult and painful urination etc.
Micke Mc Laughlin, (2000) of CSIRO, Australia has found that coffee has capacity to bind with heavy metals. Heavy metal content of water was much reduced after addition of caffeine. Dissolved heavy metal ions are positively charged and coffee contains uncharged and negatively charged molecules, the metals ions might be taken out of solution by binding to negatively charged molecules in the coffee granules.
Caffeine molecule is having a site that usually binds a divalent cation Ca++ and blocks the activity of Ca++ dependent enzyme. Caffeine has the capacity to bind with mercury. The caffeine being water soluble and common cheaper beverage, caffeine will be cheapest preventive and curative medicine. The caffeine increases the rate of urine formation and molecule of caffeine being small is easily excreted.
The alkaloid caffeine and its catabolic products theobromine and xanthine exhibit both antioxidant and prooxidant properties. Caffeine and its metabolites may also contribute to the overall antioxidant and chemo preventive properties of caffeine-bearing beverages, such as tea. (Azam et al., 2003)
Caffeine is capable of inducing certain forms of oxidative damage by increasing lipid peroxidation (Dianzani et al., 1991) Nevertheless, caffeine has been reported as a protective substance on cellular damage (Kamat et al., 2000; Krisko et al., 2005) with beneficial antioxidant effects (Nikolic et al., 2003); probably due to the main metabolites of caffeine, 1- methylxanthine and 1-methyluric acid, that are highly effective antioxidants and are able to prevent LDL oxidation in vitro (Lee, 2000).
Therefore, the present investigation carried out for the study of probable role of caffeine towards mercury.
Material and Methods
Healthy and active acclimatized bivalves of approximately same size were divided into three groups A, B and C.
(1) A group bivalves were maintained as control,
(2) B group bivalves were exposed to acute dose (LC50/2) of mercuric chloride (0.6 ppm equivalent to 0.444 ppm Hg++).
(3) C group bivalves were exposed to acute dose (0.6 ppm equivalent to 0.444 ppm Hg++) of mercuric chloride with 5 mg caffeine-l
After 4 days bivalves from group B were divided into two groups D and E.
(4) D group bivalves pre-exposed to acute dose of mercuric chloride were allowed to cure in normal dechlorinated water.
(5) E group bivalves pre-exposed to acute dose of mercuric chloride were exposed to 5 mg caffeine-l of dechlorinated water.
The experimental bivalves of A, B and C group were dissected after 24 hrs and 96 hrs and from D and E groups of recovery after 2 days and 4 days. Testis, gills and hepatopancreas from all five groups of bivalves were dried at 80 oC in an oven until constant weight was obtained. RNA contents in these tissues of control and experimental animals were estimated by using Orcinol reagent (Dischel, 1955). The results are presented in the table as percent changes of three repeats and are expressed as percentage of dry weight. Standard deviation and student’t’ test of significance are calculated and expressed in respective table.
Results
RNA contents were estimated in the gills, testis and digestive glands of freshwater bivalve L. corrianus, from the control, mercury (0.444 ppm Hg++) exposed bivalves after 24 hrs and 96 hrs with and without caffeine. And during recovery with and without caffeine from 2 days and 4 days exposed bivalves respectively and the data obtained for each biochemical with respective time of exposure from all five groups of bivalves is given in the table. The results are given in table with percent changes over control and results of statistical test.
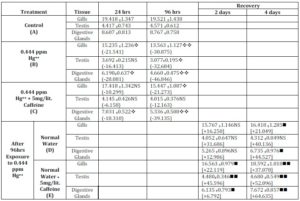
Table1: RNA Content in Selected Tissues of Lamellidens Corrianus after Acute Exposure to Hg++ without and with Caffeine and during Recovery. (Values Represent Percentage in Dry Weight)
Legends:
Values in the ( ) brackets indicate percent change over control
Values in the [ ] brackets indicate percent change over 96hrs of respective (B)
 – Compared with respective (A),
– Compared with respective (A), – Compared with respective 96hrs of (B), NS – Non Significant
– Compared with respective 96hrs of (B), NS – Non Significant
 /
/  – P < 0.005,
– P < 0.005,
 /
/ 
 – P < 0.01,
– P < 0.01,

 /
/ 

 -P < 0.001
-P < 0.001
It was observed that the control bivalves, RNA content in the gills after 24 hrs was 19.418 and after 96 hrs was 19.521. In the bivalves treated with acute concentration of mercury (0.444 ppm Hg++), the RNA content was 15.235 and 13.563 respectively for 24 and 96 hrs of exposure periods while in the bivalves exposed to mercury with caffeine (5 mg/l), the RNA content was 17.418 and 15.447 respectively for 24 and 96 hrs of exposure periods.
During recovery from mercury intoxication, the RNA content was 15.767 and 16.418 in normal water after 2 and 4 days while in normal water with caffeine (5 mg/l), the values for corresponding periods were 16.563 and 18.592.
In the control bivalves, RNA content in the testis after 24 hrs was 4.417 and after 96 hrs were 4.571. In the bivalves treated with acute concentration of mercury (0.444 ppm Hg++), the RNA content was 3.692 and 3.077 respectively for 24 and 96 hrs of exposure periods. While in the bivalves exposed to mercury with caffeine (5 mg/l), the RNA content was 4.145 and 4.015 respectively for 24 and 96 hrs of exposure periods.
During recovery from mercury intoxication, the RNA content was 4.052 and 4.312 in normal water after 2 and 4 days while in normal water with caffeine (5 mg/l), the values for corresponding periods were 4.480and 4.680.
In the control bivalves, RNA content in the digestive gland after 24 hrs was 8.607 and after 96 hrs were 8.767. In the bivalves treated with acute concentration of mercury (0.444 ppm Hg++), the RNA content was 6.190and 4.660 respectively for 24 and 96 hrs of exposure periods. While in the bivalves exposed to mercury with caffeine (5 mg/l), the RNA content was 7.031 and 5.336 respectively for 24 and 96 hrs of exposure periods.
During recovery from mercury intoxication, the RNA content was 5.265 and 6.735 in normal water after 2 and 4 days while in normal water with caffeine (5 mg/l), the values for corresponding periods were 6.135 and 7.672.
Lastly, it was observed that after acute exposure to mercury, there was a decrease in the level of RNA content in various tissues of experimental bivalves as compared to those of control bivalves. The RNA contents were higher in mercury with caffeine-exposed bivalves as compared to those exposed to only heavy metal salts. The bivalves showed the faster rate of recovery of tissue RNA level in presence of caffeine than those allowed curing naturally.
Discussion
Aquatic invertebrates naturally accumulate abnormally high amount of heavy metals. The effects of these heavy metals on the normal function of cells, tissues and organs are deleterious due to accumulative toxicity. Mercury is hazardous when accumulated even at trace level in the system of all living organisms.
RNA polymerase binds the binding site especially to its RNA template, binds its nucleotide and primer substrates, forms a new phosphodiester bond and elongates the growing RNA. Chaudhari et al., (1993) in Thiara lineata and Rao et al., (1998) in B. cunicularis observed decreased level of RNA on heavy metal stress.
Detoxification can be used as a beneficial curative measure and as a tool to increase overall health and vitality. Detoxification treatment has become one of the cornerstones of alternative medicine. Detoxification therapies are having increasing importance and popularity.
Ethylenediaminetetraacetic acid (EDTA) is often found to be chelating agent (Blaylock et al., 1997; Huang et al., 2008). EDTA is ability to chelate essential and toxic metals. Those toxicological studies that are available indicate that the apparent toxicological effects of EDTA have in fact been due to zinc deficiency as a consequence of complexation. EDTA does not appear to be teratogenic or carcinogenic in animals. The vast clinical experience of the use of EDTA in the treatment of metal poisoning has demonstrated its safety in humans WHO (2003).
EDTA in the growth medium inhibited both RNA and protein synthesis without caffeine and in the presence of lower concentration of caffeine (0.2 mM) in the growth medium, 10 micron of zinc concentration reversed RNA synthesis, which was inhibited by the chelating agent (EDTA). Higher concentrations of caffeine (2 mM) in the growth medium, completely abolished sensitivity of cardiac myocytes to zinc. Additional zinc supplementation to the growth medium of cardiac myocytes, effect of caffeine may be associated with the zinc dependent enzymes involved in RNA synthesis and caffeine zinc chelate formed makes zinc unavailable for these enzymes.
Kolayli et al., (2004) studied the binding capacities of caffeine with different micronutrients. According to him, the binding strength of the caffeine is weaker than that of the EDTA. Since all nitrogen, groups in caffeine are blocked by methylation, metals probably forms complexes with second and sixth organ of caffeine. Physical properties of bond energies suggest that oxygen metal bonds are stronger than sulfur metal bonds, hence, the caffeine molecules can drag the heavy metal that is bound to the protein.
Caffeine has capacity to bind with heavy metals. Heavy metal content of water was reduced after addition of caffeine. Caffeine binds divalent cations of calcium in Ferrete ventricular muscles (Leoty et al., 2001).
Caffeine is well-known nervous system stimulant, but besides it, it is now found that it has antioxidant activity. This activity of caffeine can protect the damage of tissue chemicals and genetic materials from heavy metal generated free oxygen radicals.
Harish et al., (2000) observed that the effect of caffeine as  reflective RNA synthesis inhibitor or given as pre-inter and post treatments on the ethyl methane sulphonate (EMS) induced adaptive responses in vivo mouse bone marrows cells was studied in order to understand the influence of caffeine on the adaptive response. Matsumura et al., (2000) studied that lack of Ca2 + and ATP dependent priming stage in caffeine induced exocytosis in bovine adrenal chromaffin cells in comparison with Ca++. They suggested that the ATP requiring priming stage is lacking in the process of caffeine-induced exocytosis in bovine adrenal chromaffin cells.
reflective RNA synthesis inhibitor or given as pre-inter and post treatments on the ethyl methane sulphonate (EMS) induced adaptive responses in vivo mouse bone marrows cells was studied in order to understand the influence of caffeine on the adaptive response. Matsumura et al., (2000) studied that lack of Ca2 + and ATP dependent priming stage in caffeine induced exocytosis in bovine adrenal chromaffin cells in comparison with Ca++. They suggested that the ATP requiring priming stage is lacking in the process of caffeine-induced exocytosis in bovine adrenal chromaffin cells.
Effect of caffeine and zinc on RNA and protein synthesis of neonatal rat cardiac mussel cell in culture was studied by Kanemaru et al., (1992) and they found that the effect of caffeine (0.2-2 mM) inhibited both RNA and protein synthesis of the cells.
Massey et al., (1993) indicated the increased urinary excretion of calcium , magnesium , sodium and chloride after oral doses of caffeine which indicates the chelated caffeine with heavy metal is excretable. In September (2001), Women’s Health Weekly also reported that, the caffeine in the drinks was primarily responsible for excess calcium excretion.
Some authors have confirmed that antioxidant effect of coffee is due to the ability to break the radical chain by donation of a hydrogen (Yen,1995; Morales and Jime´nez-Pe´rez, 2004), their affectivity as metal chelating agents (Morales et al., 2005), their capacity to reduce hydroperoxide to nonradical products (Homma and Murata,1995).
Very little work has been carried on the recovery of tissue damage and mainly caffeine’s protective role in the tissue damage by mercury. The present investigation shows the interaction of mercury and RNA. It shows that the mercury reduced the RNA contents and after the recovery with caffeine the RNA, contents were increased. The decrease in RNA on exposure to mercury may be due to damage in RNA, poor rate of synthesis of enzymes necessary for transcription or increased catabolism of RNA due to their abnormalities on binding to mercury or abnormal.
The results of biochemical estimations of RNA on acute exposure to mercury (0.444 ppm Hg++) showed drastic changes in the physiology of freshwater bivalve, L. corrianus. Mercury exposed bivalves showed decrease in the RNA contents. The exposure of mercury with caffeine showed less decrease in the contents of said biochemical as compare to those of respective mercury exposures bivalves. The faster recovery was observed after exposure to caffeine as compared to those recovered naturally in normal water.
Conclusion of this investigation that, the impact of mercury on several biochemicals, if considered as a tool for studying the toxic level, caffeine reduces the toxic stress, and hence, has a preventive and curative property against the mercury induced tissue alterations. The rapid recovery by caffeine shows that preventive role towards mercury.
Acknowledgement
The authors are thankful to Professor and Head, Department of Zoology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad for providing laboratory facilities and also thankful to Head, University Department of Interpathy Research & Technology and AYUSH Department, Maharashtra University of Health Sciences, Nashik for kind co-operation.
(adsbygoogle = window.adsbygoogle || []).push({});
References
Abbas, R. & Fisher, J. W. (1997). “A Physiologically Based Pharmaco Kinetic Model for Trichloroethelene and its Metabolites, Chloral Hydrate, Trichloroacetate, Dichloroacetate, Trichloroethonol, Glucuronid of B 6C 3F, Mice,” Toxicology and Applied Pharmacology. 147: 15-30.
Publisher – Google Scholar
Azam, S., Hadi, N., Khan, N. U. & Hadi, S. M. (2003). “Antioxidant and Prooxidant Properties of Caffeine, Theobromine and Xanthine,” Medical Science Monitor. 2003 Sep; 9(9):BR325-30.
Publisher – Google Scholar
Blaylock, M. J., Salt, D. E., Dushenkov, S., Zakharova, O., Gussman, C., Kapulnik, Y., Ensley, B. D. & Raskin, I. (1997). “Enhanced Accumulation of Pb in Indian Mustard by Soil-Applied Chelating Agents,” Environmental Science and Technology, Vol.31, 860-865.
Publisher – Google Scholar
Chaudhary, T. R., K. R. Rao, M., Subhas, S. B. Deshmukh & P. N. Patil (1993). ‘Changes in the Levels of RNA and RNA to Different Kinds of Pesticidal Stress in Thiara Lineate,’ Porc. Acad. Envion. Biol. 2 (2): 187-192.
Dianzani, M., Muzio, G., Biocca, M. & Canuto, R. (1991). “Lipid Peroxidation in Fatty Liver Induced by Caffeine in Rats,”International Journal of Tissue Reactions 13, 79-85.
Publisher – Google Scholar
Dischel, A. (1955). ‘Chemistry and Biology of Nucleic Acid Crd Chargoff and Devidson,’ Academic Press, New York.
Dushenkov, V., Kumar, P. B. A. N., Motto, H. & Raskin, I. (1995). “Rhizofilteration: The Use of Plant to Remove Heavy Metals from Aqueous Streams,” Environmental Science & Technology. 29: 1239-1245.
Publisher – Google Scholar
Falchuk, K. H., Ulpino, L., Mazus, B. & Vallee, B. L. (1977). “E. gracillis RNA Polymerase 1: A Zinc Metalloenzyme Biochem,” Biochemical and Biophysical Research Communications. 74 (3): 1206-1212.
Publisher – Google Scholar
Falchuk, K. H., Vlpino, L., Mazus, B, Ulpino, L. & Vallee, B. L. (1976). “E. gracillis RNA Dependant RNA Polymerase II: A Zinc Metalloenzyme,” Biochemistry, 15 (20): 4468-4475.
Publisher – Google Scholar
Garbisu, C. & Alkorta, I. (2001). “Phytoextraction a Cost- Effective Plant Based Technology for the Removal of Metal from the Environmental,” Bioresource Technology, 77. 229-236.
Publisher
Harish, S. K., Guruprasad, K. P., Mohmood, R. & Vasude, V. (2000). “Inducible Protective Processes in Animal Systems VI. Cross-Adaptation and the Influence of Caffeine on the Adaptive Response in Bone Marrow Cells of Mouse,” Mutagensis, Vol. 15, No. 3: 271-276.
Publisher – Google Scholar
Homma, S. & Murata, M. (1995). “Characterization of Metal Chelating Compounds in Instant Coffee,” In Sexie`mes Colloque Scientifique International sur le Cafe´; Association Scientifique International du Cafe`: Kyoto,; pp 183-191.
Publisher – Google Scholar
Huang, H., Li, T., Tian , S., Gupta, D. K., Zhang, X. & Yang, X.- e. (2008). “Role of EDTA in Alleviating Lead Toxicity in Accumulator Species of Sedum Alfredii,” Bioresource Technology, 99, 6088-6096.
Publisher – Google Scholar
Kamat, J. P., Boloor, K. K., Devasagayam, T. P. A., Jayashree, B. & Kesavan, P. C. (2000). “Differential Modification by Caffeine of Oxygen-Dependent and Independent Effects of Gamma-Irradiation on Rat Liver Mitochondria,” International Journal of Radiation Biology 76, 1281-1288.
Publisher – Google Scholar
Krisko, A., Kveder, M. & Pifat, G. (2005). “Effect of Caffeine on Oxidation Susceptibility of Human Plasma Low Density Lipoproteins,” Clinica Chimica Acta 355, 47-53.
Publisher – Google Scholar
Lee, C. (2000). “Antioxidant Ability of Caffeine and its Metabolites Based on the Study of Oxygen Radical Absorbing Capacity and Inhibition of LDL Peroxidation,” Clinica Chimica Acta 295, 141-154.
Publisher – Google Scholar
Leoty, C., Huchet- Cadiou, C., Talon, S., Choisy, S. & Hleihel, W. (2001). ‘Caffeine Stimulates the Reserve Mode of Na (SUP+) /ca (SUP2+) Exchanges in Ferret Ventricular Muscles,’ Acta physiological scandinavica, 00016772-vol, (172).
Lu, T., Liu, J., ELeCluyse, E. L., Zhou, Y.- S., Cheng, M.- L. & Waalkes, M. P. (2001). “Application of c RNA Microarray to the Study of Arsenic Induced Liver Diseases in the Population of Guizhou, China,” Toxicological Sciences 59, 185-192.
Publisher – Google Scholar
Mahajan, A. Y. (2006). ‘Certain Physiological Responses of the Freshwater Bivalve Corbicula Striatella to Heavy Metal Stress,’ Ph.D. Thesis North Maharashtra University Jalgaon.
Massey, L. K., Whiting, S. J. (1993). “Caffeine, Urinary Calcium, Calcium Metabolism and Bone,” Journal of Nutritions123 1611-1649.
Publisher
Matsumura, C., Kuwashima, H. & Kimura, T. (2000). “Lack of Ca2+ and ATP Dependent Priming Stage in Caffeine-Induced Exocytosis in Bovine Adrenal Chromaffin Cells: Comparison with Ca2+,” Journal of Autonomic Pharmacology 20(1): 31-36.
Publisher – Google Scholar
Means, J. L., Crerar, D. A. & Duguid, J. O. (1978). “Migration of Radioactive Waste, Radio Nucleotide Mobilization by Complexing Agent,” Science. 200, 1477-1481.
Publisher – Google Scholar
Morales, F. J., Ferna´ndez-Fraguas, C. & Jime´nez-Pe´rez, S. (2005). “Iron Binding Ability of Melanoidins from Food and Model Systems,” Food Chemistry., 90, 821-827.
Publisher – Google Scholar
Morales, F. J. & Jime´nez-Pe´rez, S. (2004). “Peroxyl Radical Scavenging Activity of Melanoidins in Aqueous Systems,” European Food Research and Technology, 218, 515-520.
Publisher – Google Scholar
Nikolic, J., Bjelakovic, G. & Stojanovic, I. (2003). “Effect of Caffeine on Metabolism of L-arginine in the Brain,” Molecular and Cellular Biochemistry 244, 125-128.
Publisher – Google Scholar
Prasad, M .N. V. (1997). ‘Trace Metal in Plant Ecophysiology,’ (ed. M.N.V. Prasad) John Wily and Son, New York, pp. 207-249.
Rao, K. R., P. N., Patil, T. R., Choudhari, S. R., Sasana & A. N. Vedpathak (1990). ‘Impact of Cyathion, Malathion and Endosulfan on the Rate of Respiration of the Gastropod,’ Thiara. Lineata from Panzara river. Dhule. Biol. Ind., I (II): 55-57.
Rao, T. K., Chudhary, T. R., Subhas, M. & Patil, P. N. (1998). ‘Impact of Plouride Toxicity on the Nucleic Acid Contents of the Freshwater Crabs,’ Barytelphusa cunicularis, J. Aqua. Bio. Vol. 13 (1&2) 104-106.
Saball, E., Salvarrey, M., Serra, E., Pico, G. & Ilias, M. (2000). ‘Potential Mechanism of Fibronectin Deposits in Acute Renal Failure Induced by Mercury Chloride,’ Abst. Instituto. De. Biol. Mol. Cell. De Rasario. Argentina.
Sevgi, K., Ocak, M., Kiicuk, M. & Abhasoglu, R. (2004). ‘A Study on Doses Caffeine Bind to Metal Ion,’ Elsevier, Food Chemistry, (03) P. 1-6.
Wandzilak, T. M. & Benson, R .W. (1977). “Yeast RNA Polymerase III: A Zinc Metaloenzyme,” Biochemical and Biophysical Research Communications 76 (2): 247-252.
Publisher – Google Scholar
WHO (2003). ‘Edetic acid (EDTA) in Drinking-Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality,’ Geneva, World Health Organization (WHO/SDE/WSH/03.04/58).
Yen, G.- C. & Hsieh, P.- P. (1995). “Antioxidative Activity and Scavenging Effects on Active Oxygen of Xylose-Lysine Maillard Reaction Products,” Journal of the Science of Food and Agricultur, 67, 415-420.
Publisher – Google Scholar
Zambare, S. P. & Mahajan, A. Y. (2001). ‘Heavy Metal (Copper and Mercury) Induced Alteration in the Enzyme Secretary Activity of Heoatopancrease of a Freshwater Bivalve Corbicula Striatella Poll,’ Res.(1): 143-146.



