Introduction
It had been recognized by clinicians that marked leukocytosis without any evidence of infection occasionally occurs in patients with non-hematologic malignancies. Asano et al. first demonstrated the production of human granulopoietic factors by tumor cells from a patient with lung cancer, who was associated with marked leukocytosis, and established the clinical entity of colony-stimulating factor (CSF)-producing tumor, in 1977 (Asano et al. 1977). Thereafter, 3 subtypes of CSF were identified by molecular cloning: granulocyte macrophage colony-stimulating factor (GM-CSF) (Cantrell et al. 1985), granulocyte colony-stimulating factor (G-CSF) (Nagata et al.1986), and macrophage colony-stimulating factor (M-CSF) (Kawasaki et al. 1985). In the first half of the 1990s, Suzuki et al. demonstrated that G-CSF-producing tumors produce not only G-CSF but also interleukin-1 (IL-1) and IL-6, and that IL-1 plays a role as a potent enhancer for the production of G-CSF, IL-6, and IL-1 itself (Suzuki et al. 1991), (Suzuki et al. 1992), (Tsuyuoka et al. 1994). Furthermore, Takahashi et al. showed that G-CSF-producing tumors produce IL-8 in addition to these 3 cytokines, and that such tumors are associated with marked intratumoral neutrophil infiltration, presumably due to IL-8 produced by the tumor itself (Takahashi et al. 2007). In addition, co-production of IL-1, IL-6, and IL8 in G-CSF-producing tumors appears to be caused by hyperactivation of NF-B because the expression of these 4 cytokine genes is up-regulated by this transcription factor (Takahashi et al. 2007). To our knowledge, however, there have been no reports describing IL-8-induced intratumoral neutrophil infiltration in G-CSF-producing tumors other than ours. Recently, we encountered a patient with renal cell carcinoma that produced G-CSF, IL-6, and IL-8. On pathohistological examination, marked neutrophil infiltration in the tumor tissue of surgically resected kidney was observed. We consider this neutrophil infiltration in the tumor tissue to be pathohistologically important, and thus report the serum cytokine profiles, gene expression of G-CSF and IL-8 by the renal tumor, and the histological picture of the tumor in this patient.
Case Report
A 65-year-old male was admitted to a hospital because of fever and lumbago. As the past medical history, he had hypertension and hyperlipidemia. His family history was unremarkable. Laboratory tests showed a white blood cell (WBC) count of 53.7×109/l with 88% neutrophils, a hemoglobin concentration of 7.7 g/dl, and a platelet count of 596×109/l. Serum concentration of C-reactive protein (CRP) was elevated to 15.3 mg/dl (normally below 0.3 mg). Computed tomography(CT)of the abdomen demonstrated a large mass in the upper pole of the left kidney. Therefore, he was referred to the Departments of Hematology and Urology, Shinko Hospital, Kobe, Japan, and admitted to the latter department in October 2012.
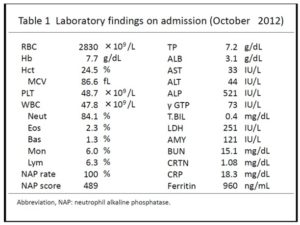
Physically, the patient was 157 cm and 51.0 kg in height and weight, respectively, with a body temperature of 37.6℃. Neither superficial lymphadenopathy nor hepatosplenomegaly was noted. Neurologic examinations were unremarkable. Laboratory tests on admission (Table 1) showed a WBC count of 47.8×109/l with 84.1% neutrophils, a hemoglobin concentration of 7.7 g/dl, and a platelet count of 487×109/l. Serum concentrations of alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (-GTP), lactate dehydrogenase (LDH), and creatinine were elevated to 521 IU/l (normally 115-360 IU), 73 IU/l (normally 0-30 IU), 251 IU/l (normally 120-230 IU), and 1.08 mg/dl (normally 0.50-1.00 mg), respectively, while those of L-aspartate aminotransferase (AST) and L-alanine aminotransferase (ALT) were almost within normal limits. The serum concentration of C-reactive protein (CRP) was markedly elevated to 18.3 mg/dl (normally below 0.3 mg). Urinalysis and urinary cytodiagnosis revealed no sign of urinary tract infection and class II (no atypia), respectively. Blood and urine cultures resulted in no growth of microorganisms.
Contrast CT scanning of the chest and abdomen demonstrated a highly necrotic mass with a major axis of 9 cm arising from the upper pole of the left kidney, which was associated with tumor embolism of the renal vein (Fig. 1A, B). Magnetic resonance imaging (MRI) of the abdomen confirmed the findings of CT scanning and showed direct tumoral invasion to the adrenal gland. These images of the tumor strongly suggested renal cell carcinoma (RCC). CT scanning and MRI also demonstrated no infectious lesion. Bone scintigraphy showed no evidence of bone metastasis.
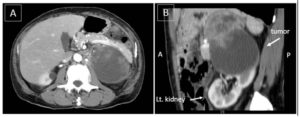
Figure 1: Contrast CT scanning of the abdomen.
A: CT scanning (axial image) at the level of the upper pole of the left kidney shows a heterogeneously enhanced mass with a major axis of 9 cm. The mass is encapsulated with fibrous tissue, resulting in a smooth surface.
B: CT scanning (sagittal image) of the left kidney. The tumor is arising from the upper pole of the left kidney.
Because of the marked leukocytosis, the presence of renal tumor, and no evidence of infection, the renal tumor was highly suspected to be CSF-producing. We thus measured serum concentrations of cytokines by enzyme-linked immunosorbent assay (ELISA). The assay was commercially performed at SRL (Hachioji, Japan). Serum concentrations of G-CSF, IL-6, IL-8, and TNF-were elevated to 474 pg/ml (normally less than 39.0 pg), 143 pg/ml (normally less than 4.0 pg), 1,260 pg/ml (normally less than 2.0 pg), and 12.5 pg/ml (normally 0.6 to 2.8 pg), respectively (Table 2). The high blood levels of G-CSF, IL-6, and TNF-α appeared to have caused the leukocytosis, high CRP, and fever in the present patient
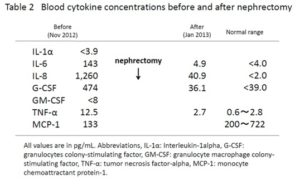
The patient subsequently underwent radical nephrectomy of the left kidney in November 2011. Intraoperatively, the renal tumor was found to have invaded the left adrenal grand and firmly adhered to the pancreas tail; therefore, resection of the left adrenal gland and pancreas tail, and splenectomy were simultaneously performed. Histopathological examination of the resected renal tumor revealed it to be stage pT3a, G3, v1, ly1 with sarcomatoid differentiation papillary RCC (Fig. 2A), and the adrenal gland-invaded tumor showed undifferentiated features of RCC, which was accompanied by marked intratumoral neutrophil infiltration (Fig. 2B, C). Interestingly, neutrophil infiltration was not prominent in the differentiated component (Fig. 2A). After the operation, the patient became afebrile, and the WBC counts and CRP levels returned to normal levels (Fig. 3). Serum concentrations of G-CSF, IL-6, IL-8, and TNF- also returned to normal or nearly normal levels (Table 2). Real-time quantitative PCR (RQ-PCR) of the tumor tissue revealed high gene expressions of G-CSF and IL-8 (described in Materials and Methods, and Results). From these findings, a diagnosis of G-CSF-producing RCC was made.
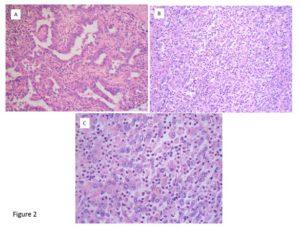
Figure 2: Histological picture of the tumor taken at nephrectomy.
A: The renal tumor tissue shows the features of relatively differentiated papillary RCC. Neutrophil infiltration is not prominent. H-E staining, ×200.
B: The adrenal gland-invaded tumor shows undifferentiated features of RCC. Marked intratumoral neutrophil infiltration is seen. H-E staining, ×200.
C: Higher magnification (×400) of the same tumor (H-E staining).
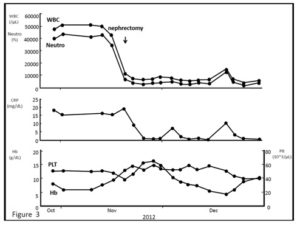
Figure 3: Changes of laboratory data before and after nephrectomy. High WBC/neutrophil counts and high serum CRP levels before the nephrectomy returned to normal levels.
The patient, however, had to stay in hospital because he developed aspiration pneumonia, adhesive ileus, recurrent esophageal ulcer, and cerebral infarction. In June 2013, serum enzyme levels of the biliary tract and then those of transaminases increased, and he became icteric one month later. He developed cancerous peritonitis and CT scanning showed metastatic lesions in the diaphragm. The WBC count increased to 483×109/l with 93% neutrophils, indicating the recurrence of the G-CSF-producing tumor. The patient died at the end of August, 9 months after the operation.
Materials and methods
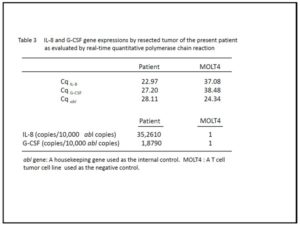
Total RNA was extracted from resected tumor tissue and a T-cell tumor cell line, MOLT4, as a negative control, using RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). One microgram of total RNA was reverse-transcribed by SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, California, USA). For G-CSF and IL-8 gene, RQ-PCR was performed using Sso Fast EvaGreen Supermix (Bio-Rad, Mississauga, ON, Canada). The ABL gene was chosen as a housekeeping gene to evaluate the amount and amplification of cDNA. The gene-specific primers used were 5’-AAGCTGGTGAGTGAGTGTGC-3’ and 5’-ATGGAGTTGGCTCAAGCAGC-3’ for G-CSF, 5’-GGTGCAGTTTTGCCAAGGAG-3’ and 5’-TTCCTTGGGGTCCAGACAGA-3’ for IL-8, 5’-TGGAGATAACACTCTAAGCATAACTAAAGGT-3’ and 5’-GATGTAGTTGCTTGGGACCCA-3’ for ABL. The quantification of each gene expression was performed according to the method by Østergaard et al (Østergaard et al. 2004). To quantify the individual expression levels, we measured quantification cycles (Cq) for these genes, in which the fluorescence intensity of each PCR product reached the same level (Table 3). Then, we calculated the expression levels of each gene per 10,000 copies of abl gene according to the following formula:10,000×2^(CqABL–CqTARGET), assuming PCR amplification efficiency as 2.
Results
As shown in Table 3, the expression levels of IL-8 and G-CSF were 352,610, and 18,790 times of that of MOLT4, respectively.
Discussion
Regarding the diagnosis of G-CSF-producing tumor, the present case fulfills the criteria proposed by Asano et al., that is, marked granulocytosis, elevated serum level of G-CSF, normalization of granulocyte count and serum G-CSF level following tumor resection, and demonstration of G-CSF production by the tumor. As for the organ of origin of the G-CSF-producing tumor, it varies and includes the lung (Asano et al. 1977), (Nakamura et al. 1997), stomach (Tahara 1990), pancreas (Joshita et al. 2009), thyroid (Kang K et al. 2013) (Vassilatou et al. 2006), gingiva (Kobayashi et al. 2012), kidney (Wang et al. 2006), (Sugiura et al. 2007), (Kanda et al. 2011), bladder (Ito et al. 1990), and ovary (Münstedt et al. 2010).
G-CSF-producing RCC is relatively rare among renal cancers, and being associated with a poor prognosis (Wang et al. 2006). Currently, no standard and effective treatment is available for this type of tumor (Wang et al. 2006). To our knowledge, however, radical nephrectomy and/or combination chemotherapy with gemcitabine and nedaplatin (Sugiura et al. 2007), or that with sorafenib and S-1 (Kyono et al. 2011) are potentially effective treatment options.
Regarding IL-8 production by malignant tumors, Takahashi et al. described that there are 2 types: one is IL-8 production in the multi-cytokine production of G-CSF-producing tumors like the present case, and the other is sole IL-8 production by tumors (Takahashi et al. 2007), although the degrees of intratumoral neutrophil infiltration are similar in these two types of tumor (Takahashi et al. 2007). In terms of the important differences between these 2 types, the latter patients are afebrile and exhibit normal CRP levels and mild leukocytosis, presumably caused by IL-8-activated neutrophil mobilization from the marginal pool, but not enhanced granulopoiesis as in G-CSF-producing tumors (Takahashi et al. 2007), (Terashima et al. 1998).
In relation to neutrophil infiltration in tumor tissue, biliary system- dominant liver dysfunction was observed at presentation and during the recurrence of the tumor in the present patient. Furthermore, the patient developed marked jaundice with direct bilirubin dominance. Drug-induced liver dysfunction was ruled out. Suzuki et al. reported liver damage in patients with G-CSF-producing tumor (Suzuki et al. 1993). In the postmortem examination of the liver, neutrophils infiltrated both the portal area and the centrilobular zone even in patients without liver metastasis, and the authors speculated that the G-CSF- and presumably IL-8-activated neutrophils which passively infiltrated the liver and accumulated themselves in the above mentioned areas, caused the liver damage. We speculate on a similar event caused by infiltrated neutrophils in the liver because the pattern of liver dysfunction was biliary system-dominant, as in patients in the study by Suzuki et al. (Suzuki et al. 1993).
G-CSF-producing tumors are associated with a poor prognosis. For example, there have been no patients who survived for more than 1 year after diagnosis in G-CSF-producing tumors of the urinary tract (Sugiura et al. 2007), (Kanda et al. 2011), (Ito et al. 1990). Regarding the poor prognosis, Gregory et al. discussed the relationship between tumor growth and neutrophil infiltration and proposed the concept of tumor-associated neutrophils (TAN) (Gregory et al. 2011). Although they discussed TAN in non-CSF-producing tumors, the relationship between tumor growth and neutrophils appears also to occur in G-CSF- and IL-8-producing tumors. In terms of the mechanism by which TAN confer an advantage for tumor growth, they proposed several factors: first, infiltrated neutrophils secrete oncostatin M that makes tumor cells produce vascular endothelial growth factor or hepatocyte growth factor, which subsequently enhances tumor invasion or growths. Second, neutrophils produce reactive oxygen species that induce tumor transformation through DNA injury and repair. Third, IL-8-activated neutrophils promote tumor metastasis through their adhesion to vascular endothelium and subsequent expression of integrin by endothelial cells, which leads to the adhesion of the tumor cells and extravascular tumor invasion. Fourth, neutrophil elastase destroys insulin receptor substrate, which leads to phosphoinositide 3-kinase activation and tumor growth. In relation to TAN, Bellocq et al. and Jansen et al. observed that the presence of intratumoral neutrophils is associated with a poor prognosis in bronchioloalveolar carcinoma and localized RCC, respectively (Bellocq et al. 1998),(Jensen et al. 2009).
In conclusion, physicians should be aware of intratumoral neutrophil infiltration, which is caused by IL-8 production by the tumor, and biliary system-dominant liver dysfunction, which may be caused by intrahepatic neutrophil infiltration in G-CSF-producing tumors.
(adsbygoogle = window.adsbygoogle || []).push({});
References
- Asano, S., Urabe, A., Okabe, T., Sato, N., Kondo, Y. (1977) “Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue”, Blood, 49 (5) 845-852.
Google Scholar
- Bellocq, A., Antoine, M., Flahault, A., Philippe, C., Crestani, B., Bernaudin, J. F., Mayaud, C., Milleron, B., Baud, L., Cadranel, J. (1998) “Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome,” The American journal of pathology, 152(1) 83-92.
Google Scholar
- Cantrell, M. A., Anderson, D., Cerretti, D. P., Price, V., McKereghan, K., Tushinski, R. J., Mochizuki, D. Y., Larsen, A., Grabstein, K., Gillis, S., Cosman, D.(1985) “Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor,” Proceedings of the National Academy of Sciences of the United States of America, 82 (18) 6250-6254.
Google Scholar
- Gregory, A. D., Houghton, A. M. (2011) “Tumor-associated neutrophils: new targets for cancer therapy,” Cancer research, 71(7)2411-2416.
Google Scholar
- Ito, N., Matsuda, T., Kakehi, Y., Takeuchi, E., Takahashi, T., Yoshida, O. (1990) “Bladder cancer producing granulocyte colony-stimulating factor,” The New England journal of medicine, 323(24)1709-1710.
Google Scholar
- Jensen, H. K., Donskov, F., Marcussen, N., Nordsmark, M., Lundbeckm, F., von der Maase, H. (2009) “Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma,” Journal of clinical oncology, 27(28)4709-4717.
Google Scholar
- Joshita, S., Nakazawa, K., Sugiyama, Y., Kamijo, A., Matsubayashi, K., Miyabayashi, H., Furuta, K., Kitano, K., Kawa, S. (2009) “Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course,” Internal Medicine, 48 (9) 687-691.
Google Scholar
- Kanda, S., Inoue, T., Tsuruta, H., Chiba, S., Obara, T., Saito, M., Kumazawa, T., Tsuchiya, N., Satoh, S., Habuchi, T. (2011) “Granulocyte colony stimulating factor-producing spindle cell renal cell carcinoma successfully treated by chemotherapy consisting of gemcitabine and doxorubicin,” Hinyokika Kiyo, 57(7)385-389. (In Japanese with English abstract)
Google Scholar
- Kang, K., Park, J. H., Ryu, J. Y., Lee, S. Y., Ko, G. J., Kwon, Y. J. (2013) “Acute pyelonephritis with anaplastic thyroid carcinoma producing granulocyte colony-stimulating factor,” Blood research, 48(1) 63-66.
Google Scholar
- Kawasaki, E. S., Ladner, M. B., Wang, A. M., Van Arsdell, J., Warren, M. K., Coyne, M. Y., Schweickart, V. L., Lee, M. T., Wilson, K. J., Boosman, A., Stanley, E.R., Ralph, P., Mark, D. F.(1985) “Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1),” Science, 230 (4723) 291-296.
Google Scholar
- Kobayashi, J., Miyazaki, A., Yamamot, T., Nakamori, K., Suzuki, R., Kaneko, T., Suzuki, N., Hiratsuka, H. (2012) “Granulocyte colony-stimulating factor-producing squamous cell carcinoma of the lower gingiva: a case report,” Head & neck oncology, 4, 35.
Google Scholar
- Kyono Y., Takayama T., Kinoshita M., Kurita M., Mugiya S., Baba S., Ozono S. (2011) “Combination therapy with sorafenib and S-1 for renal cell carcinoma producing granulocyte colony-stimulating factor”, International Journal of Clinical Oncology, 16(3)275-278.
Google Scholar
- Münstedt, K., Hackethal, A., Eskef, K., Hrgovic, I., Franke, F. E. (2010) “Prognostic relevance of granulocyte colony-stimulating factor in ovarian carcinoma.” Archives of gynecology and obstetrics. 282(3)301-305.
Google Scholar
- Nagata, S., Tsuchiya, M., Asano, S., Kaziro, Y., Yamazaki, T., Yamamoto, O., Hirata, Y., Kubota, N., Oheda, M., Nomura, H., Ono, M. (1986) “Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor,” Nature, 319 (6052) 415-418.
Google Scholar
- Nakamura, M., Oshika, Y., Abe, Y., Ozeki, Y., Katoh, Y., Yamazaki, H., Kijima, H., Ueyama, Y., Ogata, T., Tamaoki, N. (1997) “Gene expression of granulocyte colony-stimulating factor (G-CSF) in non-small cell lung cancer,” Anticancer research, 17 (1B) 573-576.
- Østergaard, M., Olesen, L. H., Hasle, H., Kjeldsen, E., Hokland, P. (2004) “WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients – results from a single-centre study,” British journal of haematology, 125 (5) 590-600.
Google Scholar
- Sugiura, S., Makiyama, K., Nakaigawa, N., Yao, M., Kubota, Y., Oshiro, H. (2007) “Collecting duct carcinoma producing granulocyte-colony-stimulating factor (G-CSF),” International journal of urology, 14(6):555-557.
Google Scholar
- Suzuki, A., Takahashi, , Okuno, Y., Nakamura, K., Tashiro, H., Fukumoto, M., Konaka, Y., Imura, H. (1991) “Analysis of abnormal expression of G-CSF gene in a novel tumor cell line (KHC287) elaborating G-CSF, IL-1 and IL-6 with co-amplification of c-myc and c-ki-ras,” International journal of cancer, 48 (3) 428-433.
Google Scholar
- Suzuki, A., Takahashi, T., Okuno, Y., Tsuyuoka, R., Fukumoto, M., Nakamura, K., Imura, H. (1992) “IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines,” British journal of cancer, 65 (4) 515-518.
Google Scholar
- Suzuki, A., Takahashi, T., Okuno, Y., Seko, S., Fukuda, Y., Nakamura, K., Fukumoto, M., Konaka, Y., Imura, H. (1993) “Liver damage in patients with colony-stimulating factor-producing tumors,” The American journal of medicine, 94(2)125-132.
Google Scholar
- Tahara, E. (1990) “Growth factors and oncogenes in human gastrointestinal carcinomas”, Journal of cancer research and clinical oncology, 116 (2) 121-131.
- Takahashi, T. and Kasakura, S. (2007) “Inflammatory Markers and Cancer. XXIV World Congress of Pathology and Laboratory Medicine,” MEDIMOND S. r. l., Bologna, (2007)77-82.
- Terashima, T., English, D., Hogg, J. C., van Eeden, S. F. (1998) “Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8,” Blood, 92(3) 1062-1069.
Google Scholar
- Tsuyuoka, R., Takahashi, T., Sasaki, Y., Taniguchi, Y., Fukumoto, M., Suzuki, A., Nakamura, K., Kobayashi, S., Kudo, T., Nakao, K. (1994) “Colony-stimulating factor-producing tumours: production of granulocyte colony-stimulating factor and interleukin-6 is secondary to interleukin-1 production,” European journal of cancer, 30A (14) 2130-2136.
Google Scholar
- E., Fisfis. M., Morphopoulos. G., Savva. S., Voucouti. E., Stefanoudaki. K., Tzavara. I. (2006) “Papillary thyroid carcinoma producing granulocyte-macrophage colony-stimulating factor is associated with neutrophilia and eosinophilia.” Hormones (Athens), 5(4)303-309.
Google Scholar
- Wang, Y. C., Yang. S., Tzen., C. Y., Lin, C. C., Lin, J. (2006) “Renal cell carcinoma producing granulocyte colony-stimulating factor.” Journal of the formosan medical association, 105(5)414-417.
Google Scholar








