Introduction
Dietary manipulations can be achieved by reduction in their sulfur, protein and indigestible components. Consequently, substrates that are available for the odor producing microbes are removed. The inclusion of dietary enzymes, low fiber feedstuffs and mineral additives can also mitigate odor matter production as reported by Hobbs et al., 1996; Obrock et al., 1997; Sutton et al., 1998; Kempen and Heugten, 2005; Chen et al. 2005. Differences in environmental and physiological conditions were confirmed by Whitehead and Cotta (2001) to significantly influence fecal microbes. To date studies on the relationship of dietary protein manipulation to seasonal change on pig fecal microflora and emission of odorous compounds are few and limited in scope. According to Hover (2005), when there is an excess dietary protein, the undigested residues of the dietary protein can reach the distal part of the ileum and enhance the risk of proliferation of pathogens such as E. coli and Clostridium pathogens. Murry et al. (2002) were able to detect increased in fecal lactic acid bacteria and emission trend for fecal p-cresol, skatole, indole and phenols when a diet of growing pig was supplemented with 0.3% lacto sucrose. Simpson et al. (2002) characterized fecal bacterial population in dogs while Yen et al. (2004) in pigs with high dietary fiber supplementation. The fecal enterotoxigenic E. coli (K88) was also enumerated by Owasu-Asiedu et al. (2003) in weanling pigs fed diet supplemented with spray-dried porcine plasma, or pea protein isolate plus egg yolk antibody. Merril and Halverson (2002) reported that changes in abundance of microbial species are expected during summer than any other seasons. Likewise Hobbs et al. (1996) observed a reduction in odor compounds when pigs were fed diets low in protein. When these workers compared diets high in protein (208 gkg-1, grower: 189 gkg-1, finisher) and low in protein (161 gkg-1, grower: 138 gkg-1 finisher), the concentration of compounds measured was lower in the low-crude protein diets during the grower period.
The presence of microbes in the feces is an indication that there is a substantial amount of organic matter which when decomposed results in the production of odorants (Whittington and Lemay, 2006). On the other hand, Claus et al. (2003) investigated the effect of high potato starch, which can highly influence fecal organic matter and skatole. New emerging evidence also suggests that odorous compounds arise from the incomplete anaerobic degradation of carbohydrates, fatty acids and protein (Zhu and Jacobson, 1999). In contrast, dietary modification with a specific structure of carbohydrates known as fructooligosaccharides in piglet diet was not able to control odor as there was a significant increase in fecal total volatile fatty acids (Xu et al., 2005). Ritter (1989) claimed a high concentrated malodor emission during winter due to sludge build up. The concentration of gram-negative bacteria in the winter sampling series exceeded the 103 CFU/m3 limit recommended by Malmros (1990) and Malmros et al. (1992) for waste-treatment and composting facilities. During spring, malodor is also evident due to increased bacterial action (Hamilton and Cumba, 2000). The mixture of microorganisms, comprised of bacteria and actinomycetes, has the ability to rapidly deodorize and compost feces from pigs as demonstrated by Ohta and Ikeda (1978). Pig manure odor offensiveness did not diminish and fecal microbial concentrations remained constant when dietary protein was reduced from 15% to 9% as reported by Otto et al. (2003)
However, the above measurements were done in manure stored in lagoons. Therefore, quantifying malodor directly in feces is important to get a more accurate assessment of their varying emission levels; hence, this investigation pursued this as one of its research objectives. Fecal microbe enumeration was also necessary to relate its kinetics relative to both DPL and seasonal variation, wherein there is a dearth of information on this research knowledge.
Materials and Methods
Animals, dietary treatments and management practices
A total of 36 grower pigs, Landrace, with an average initial weight of 25kg were used in the study. The growers were blocked based on their body weight and within each block assigned to one of the three dietary treatments (3 replicates (pens) per treatment) with 2 males and females per pen. The growers of different mean weight groups, 25, 32 and 40 kg were offered diets containing three dietary protein levels, 142.7, 155.4 and 174.9 gkg-1 in a 3 x 3 factorial arrangement during the time of fecal microbe collection. On the other hand, the finisher’s mean weight at the time of fecal microbe sampling was 52, 56 and 69 kg, respectively and their DPLs were 97.3, 106.3 and 116.9gkg-1, respectively. All dietary treatments were fed in mash form. The corn based grower dietary treatments had a common DE value of 3500 kcalkg-1 and other nutrient requirements for each particular DPL were based on NRC (1998) (Table 1). The pigs were housed in a concrete slatted floor pen beneath pit slurry with a floor space area of 4 m2. Each pen was provided with a bowl type nipple drinker and semi-automatic feeding trough.
Feces sampling for microbial counts and odorants
Fresh 400g fecal samples were collected from newly defecated pig represented in the different treatment replicates. Collection was done for three consecutive times at bi weekly interval. All feces were picked up using individual and brand new plastic hand gloves to prevent cross contamination of collected fecal samples.
After transferring to the laboratory, mashing and thorough mixing the pooled feces on the sterilized stainless pan, each representative sample from them was collected for microbial counts, 10g, and for fecal odorants, 250g in the clean bench, respectively. The incubation conditions, where the different culture media, temperature requirement and duration are specified for microbial enumeration, are presented in Table 2.
Microbial counts in feces
For the purpose of detection of the microbial population in feces, a series of ten-fold dilutions (10-1 to 10-10) with 1% peptone water (pH 6.8) using the methods of APHA (1998) was made in aerobic diluents. From appropriate dilutions, 0.05 ml aliquots were spread onto five selective agar plates and counted the colony on the agar plates by Quebec colony counter; BCP plate count agar (Manufacture: Eiken, gL-1, Yeast extract 2.5g, Peptone 5g, Glucose 1g, Tween80 1g, L-Cysteine 0.1g, Bromo cresol 0.04g, Agar 15g, adjust to pH PH6.9) for lactic acid bacteria, Chromocult agar (Manufacture: Merck, gL-1, Peptone 3.0g; Sodium chloride 5.0g; Sodium dihydrogenphospat 2.2g; Di-sodium hydrogenphospat 2.7g; Tryptophan 1.0g; Sodium pyruvate 1.0g; Tergitolዊp7 0.15g; Sorbitol 1.0g; Chromogenic mix 0.4g; agar 10.0g, adjust to pH:6.8ዊp0.2) for E. coli, coliform, Gram negative bacteria and total Enterobactericiae, Tryptic soy agar (Manufacture: Difco, gL-1, Tryptone Peptone pancreatic digest of casein, 15.0g, Soytone peptone papaic digest of soybean meal 5.0g, Sodium chloride 5.0g, agar15.0g, adjust to pH 7.3±0.2) for total bacteria, Potato dextrose agar (PDA, Manufacture: Difco , gL-1, Potato Starch 4.0g, Dextrose 20.0g, agar15.0g, adjust to pH 5.6± 0.2) with tartaric acid for fungi, and SS agar (Manufacture: Difco, gL-1, Beef Extract 5.0g, Proteose Peptone 5.0g, Lactose 10.0g, Bile Salts No.3 8.5g, Sodium Citrate 8.5g, Sodium Thiosulfate 8.5g, Ferric ammonium citrate 1.0g, Neutral Red 0.025g, agar 13.5g, Brilliant green 0.33mg, adjust to pH 7.0±0.2) for Salmonella, spp.
The identification of the bacteria and fungi were performed based on colonial and cellular morphologies, Gram-reaction, spore formation, and aerobic growth on the above media. The bacterial counts are expressed as the log10 of a CFU per gram weight of fecal samples. Using the methods and media (Table 2) of Mitsuoka et al. (1976), Terada et al. (1992), Frampton et al. (1988), Manafi and Kneifel (1989), and Kilian and Bülow (1976), the fecal flora was analyzed.
Measurement of malodorous compounds
For the purpose of detection for odorants emitted from the feces, the feces were incubated at 25 ˚C for 6 days. The various odorants investigated in the present study were; iso-valeraldehyde, valeraldehyde, methyl iso-butyl ketone, butanol and xylene. The trapped odorants were analyzed form the feces collected from the pens of different pigs represented in the different treatment replicates. The odorants were measured by Gas chromatography-Mass (Model; Saturn GC/MS WS Ver. 5.4, Manufacturer; Varian, USA) after placing around 250 grams of the collected feces to an in vitro glass flask to where the odorants were trapped at 25 ˚C and incubated for 6 days.
For the detection of malodorants emitted from feces, the malodorants were collected into a canister (SilcoCanTM, 110 Benner Circle Bellefonte, PA 16823) during the incubation and coupled with the GC/MS according to the standard protocol of the Gas chromatography-Mass (Model; Saturn GC/MS WS Ver. 5.4, Manufacturer; Varian, USA). The malodorants were analyzed with a Varian Saturn GC/MS equipped with a CP WAX 52CB DF=1.2um capillary column. The details of the gas chromatograph conditions are as follows: carrier gas, helium; split ratio, 10:1; FID helium, 1.0 ml/min; FID nitrogen, 10 ml/min; initial temperature, 30ዊ holding for 7min; program rate, 6ዊ /min holding for 10min; final temperature, 200ዊ holding for 10min; injection port, – 100ዊ holding for 4min. Malodorants’ profiles were analyzed by GC-2000.40 software (Varian, U.S.A.).
The details of the Mass (Model 3800, Varian, USA) conditions are as follows: temperature; ion trap 180ዊ, manifold 40ዊ, Xferline 200ዊ, Ion value; 500, RF Dump value; 300 m/z, Scan range; 42~300m/z, Scan rate; 0.6 second/scan, Background mass; 42m/z, Target; 20000 counts, and Emission current; 10 uA. Malodorants’ profiles were analyzed by MS-3800.44 software (Varian, U.S.A.).
Statistical Analysis
The experiment was conducted to determine the effect of protein level and weight on fecal chemical composition. The data gathered were analyzed in one-way weight on fecal chemical composition. The data gathered were analyzed in one-way ANOVA of SPSS version 10 (SPSS Inc. Chicago, USA, 1999). Comparison among means was done in Duncan Multiple Range Test at 5% level of significance.
Results
Fecal microbe shedding as influenced by dietary protein and season
During the growing stage, as shown in Table 3, the feces of growing pigs fed with varying DPLs were enumerated for pathogenic and non-pathogenic microbes. The varying DPLs used in this experiment did not influence changes in fecal total bacteria, Salmonella spp, E. coli, other gram negative bacteria, total enterobactericiae and fungal population in feces
In contrast, the kinetics of fecal microbial shedding for finishing pigs varied greatly with the growing pigs as demonstrated in Table 4.
Fecal odorous compounds as influenced by dietary protein and season
The effect of DPL (bodyweight) and season on iso-valeraldehyde emission is presented in Table 5. The maximum level of iso- valeraldehyde concentration in this investigation was 3.9 mgL-1
In Table 6, the valeraldehyde emission from the pig barn regardless of DPL ranged from 5.85 to 44.15 mgL-1 while during late summer it was detected from 53.4 to 68.7 mgL-1.
Reducing or increasing DPL or body weight did not result in any definite trend or changes in emission of methyl isobutyl as presented in Table 7.
As shown in Table 8, DPLs did not also have a marked influence on butanol emission. However, there was also a tendency to increase at lower DPLs during late spring and late summer.
With the varying levels of protein substrates as our other variable, we found out that despite differences in season and DPLs, butyric acid concentration remained comparable among treatments however there was undetectable level of this malodorant in the pens of finishing pigs offered the varying protein levels during late summer as shown in Table 9.
In Table 10, finishing pigs fed a reduced DPL of 97.3gkg-1 during the late summer had a significantly lower xylene emission than the 106.3gkg-1 DPL.
In the present study, toluene emission was likewise unaffected by DPL, body weight and seasonal change as shown in Table 11.
Discussion
Fecal microbe shedding as influenced by dietary protein and season
There was no definite trend in decrease or increase in population in the fecal microbe shedding as influenced by dietary protein and season. However, the investigation noted that fecal coliform count of pigs offered the highest dietary CP of 174.9gkg-1 had a significant increase (p<0.75) from 2.7 x 103 to 6.3 x106 CFU/g from mid spring to early summer or a period of 28 days.
Fecal salmonella, coliform other gram negative bacteria and total enterobacteria were not influenced by DPLs from mid summer to early autumn. Moreover, there was no characteristic pattern of growth in the other microbes investigated. Van Heugten et al. (2003), on the other hand, observed increased fecal lactobacilli and coliform counts in pigs fed diets supplemented with antibiotics and high levels of Cu. Similarly, Sutton et al. (1999) reviewed that lacitol, yeast, and Lactobacillus acidophilus reduced the odorous compounds, indole and skatole. The above findings indicated the varying responses of fecal microbe dynamics by different variables or management interventions. Holloway (2002) reported that aerobic bacterial degradation, if complete, converts organic compounds such as amino acids and carbohydrates to CO2 resulting in lesser production of odorous compounds. In our investigation, the fecal concentration of aerobic bacteria decreased (p < 0.090) from 1.5 x 109 to 2.93 x 10 8 when dietary protein was reduced from 116.9 to 106.3gkg-1 during mid summer season indicating that DPL influenced the fecal odor generation during this period.
The concentration of endotoxin in the air of pig buildings is recognized as particular hazard to pig workers as claimed by Martin et al. (2005). Interestingly, the endotoxic activity from E. coli was the most potent as reported by Hurley (1995). E. coli is derived exclusively from feces and manure (JEQ Executive Summaries, 2005). The fecal E. coli of pigs offered the lowest CP of 9.73 % had the most pronounced increase (p < 1.0) from 6.2 x 10 5 to 1.0 x 10 8 CFU/g, which was observed from mid summer to early autumn. This can therefore be a major concern of the study as a low dietary CP during finishing stage could be detrimental. However in the growing stage, fecal E. coli counts remained stable indicating that higher dietary protein has a suppressing effect on population growth. Fecal lactic acid bacterial colony count also increased significantly from pigs offered the 11.2 and 12.3 %, during early autumn. Pigs fed a lower DPL of 103 gkg-1 were able to maintain only their low fecal lactic acid colony count, which is an indication that this protein level cannot depress malodor in feces. Formulating diets less than 112 gkg-1, therefore, will be detrimental as far as odor control is concerned.
Exposure to mold spores has been associated with allergic alveolitis and toxic organic dust syndrome by Eduard et al. (1993). In the present study, our low DPL of 97.3 gkg-1 for finishing hogs cannot depress fecal fungal colony count while the dietary protein levels of 106.3 and 116.9 gkg-1, respectively, remained unchanged although they tended to decrease during early autumn.
Fecal odorous compounds as influenced by dietary protein and season
High doses of iso-valeraldehyde (CH3-CH(CH3)-CH2-CHO), which is an irritating odor with obnoxious odor, can penetrate the body through oral, dermal and inhalation. It is soluble in water at 20,000 mgL-1 under 20 °C and has a vapor pressure of 6100 Pa at 20°C (SIDS, 2000). The DPLs and body weights did not have a pronounced effect on changes in the emission of iso– valeraldehyde. However, there was a tendency of this malodorant from the pens of the pigs offered the lowest DPL to be undetectable. Iso-valeraldehyde has a low toxicity level and but can be irritating to the eyes. Salem and Cullumbine (1960) reported an acute inhalation toxicity of 6.2 mgL-1 for 10 h. and therefore was considered safe. It must be considered that odorant detection was done for a 6-day fecal incubation period due to the fact that odorous nature of manure increases after 5 days of storage Jacobson et al. (2001). Accordingly, the odor concentration obtained in this investigation can be used to approximate the odorous nature of the feces under natural emission condition. Therefore, if direct measurement of this odorous compound is done, it can still be substantially reduced further. In Korea, the industrial permissible standard for iso– valeraldehyde is 0.003 ~ 0.006 mgL-1 (Yang et al. 2005).
Valeraldehyde (O=CH-CH2-CH2-CH2-CH3) is a colorless liquid with a strong, pungent odor. Because of its low flash point, it is a dangerous fire hazard (ACGIH, 1999). It is one of the major odorants responsible for odor emissions. It can be neutralized by the addition of ash coming from wood as reported by Rosenfield et al. (2002). They further added that ash could neutralize odor due to its high alkalinity. In our previous experiment we noted increasing fecal ash content on finishing pigs fed a protein level of 97.3 gkg-1 indicating lesser fecal-based odorants. The potential for odor production is dependent on the physical characteristics of the feedstocks to which feces is included. Ash was also emphasized by Goldstein (2002) as a quick mitigating measure for odor control.
The reported odor thresholds of valeraldehyde are 0.028 and 0.10 mgL-1 (Amoore and Hautala 1983; Johansson et al. 1973). In our investigation the emission was unaffected by the DPLs in both the growing and finishing stages, however, there was a significant increase in the emission level from early summer to late summer when the protein levels of the finishing hogs were reduced from 155.4 to 106.3 and 174.9 to 116.9gkg-1, respectively. At the time of measurement, the approximate temperature during early summer inside the pig barn was 22 ˚C while late summer was 25 ˚C. Apparently, this indicated the combined effects of temperature, season and DPLs in influencing significant increase in valeraldehyde emission.
On the other hand, the occupational exposure limits for valeraldehyde in most countries such as the Netherlands and Denmark is 50 pm at a time-weighed average of 8 hrs (CUOEL. 2003). Therefore, there is a need for pig farmers to improve pig barn ventilation during late summer or institute malodorant reduction strategies during this period. Interestingly, there was a tendency for this malodorant to increase at higher DPLs.
Butanol (C4H9OH) has a weak fuel oil odor (Merck Index, 1968). As such, it is usually the most commonly used reference odorant, which is readily available in high purity relatively non toxic, stable, and has a reasonably agreeable odor that is unrelated to most other odors of interest (Mackie et al. 1998).
And also, butanol being a weak odorant could still be detected across season. This is an indication that should the variation in DPL be greater, this odorant can be a major concern to livestock farmers as far as their prolonged exposure is concerned. Follow up investigations on further dietary manipulation and its mitigating effect on odor emission should therefore be encouraged.
Carbohydrates through bacterial fermentation are converted to volatile fatty acids such as acetic, propionic, and butyric acid. When they are excreted with the feces, they can contribute to odour with butyric acid having the most unpleasant odor (Van Kempen and Van Heugten, 2003). There are many variables resulting in varying kinetics of fecal butyric acid emission. Chen et al. (2005) noted that by supplementing the diet of finishing pigs with clay minerals, the fecal butyric acid concentration decreased significantly and has more dramatic effect in high protein diets. On the other hand, the butyric acid level in weanling pigs fed a high dietary protein of 20 % was not influenced by either antibiotic medication or supplementation of essential oils as reported by Cho et al. (2006). On the other hand, Chen et al. (2006a) demonstrated no increase in fecal butyric emission from finishing pigs fed Enterecoccus – based probiotics. However, their follow up experiment using Bacillus – based probiotics in finishing pig resulted in a significant decrease in fecal butyric emission (Chen et al, 2006b). It had a concentration of 0.22 mgL-1 to 3.27 mgL-1 during the growing stage whilst it was zero level during the finishing stage. This indicated that protein levels ranging from 97.3 to 116.9gkg-1 seemed to be effective in suppressing butyric acid concentration when finishing pigs were offered these varying DPLs that were below their requirement.
Xylenes are classified as moderate toxic air contaminants (SDB.2001). During late spring, there were undetectable levels of xylene from all the pig pens fed with the different DPLs indicating no influence of nutrient changes on this odorous compound. Among the malodorants measured in this experiment, xylene was significantly reduced when the DPL was likewise decreased during its collection in late summer. This indicated that xylene concentration could be influenced by dietary manipulation and a function of season and its volatile nature. Epidemiological studies suggest that chronic occupational exposure to 14 mgL-1 can increase symptoms such as headaches, eye and nasal irritation, and a floating sensation. Some animal studies suggest that exposure to levels greater than 241 mgL-1 during gestation can retard fetal growth and cause skeletal abnormalities (OME, 2001). Our investigation in an enclosed pig barn with nutritional and seasonal interventions demonstrated that this odorant that we trapped in the feces, which has a concentration of 0-4.36 mgL-1 was very much lower than the hazardous exposure level for pigs. Xylene is a member of a class of volatile organic chemicals that react with oxides of nitrogen in the presence of sunlight to form ground-level ozone, a component of smog. Xylene does not contribute to global warming. Volatilization and microbial degradation are the major Canada (1993) concluded that xylene is not expected to persist or bioaccumulate in the environment.
Toluene is a colorless liquid with a sweet, strong odor and is used as a solvent in aviation gasoline. It should be treated with extreme caution as it is considered as a teratogen which can cause brain and kidney damage. NJDHSS (1998) reported the legal airborne permissible exposure limit (PEL) of this odorant to be 200 mgL-1 averaged over an 8-hour work shift, 300 mgL-1 averaged over an 8-hour work shift, 300 mgL-1 not to be exceeded during any 15 minute work period and 500 mgL-1 as a 10 minute acceptable maximum peak. It is reassuring however that the maximum detection level for toluene in the present study was 0.89 ugL-1, which was very much lower than the different hazardous concentrations mentioned above.
However, there was a tendency for it to be at 0 mgL-1 concentration in the lowest DPL of 97.3gkg-1 in the finishing stage of the pigs belonging to the lightest weight group of 55.98 kg.
Conclusions
This investigation was able to identify fecal microbes and odorants from a specific growth stage of hogs given varying DPLs. Likewise, documentation was done on their kinetics relative to diet and seasonal change. Accordingly, these benchmark data can be considered useful in further studying the metabolism of these microbes in producing odorous compounds. Finally, this will result in a better understanding of malodorants and the underlying factors leading to their origin. As a result more effective malodorants and the underlying factors leading to its origin. As a result more effective recommendations can be done in the future.
Acknowledgement
This study was supported by the Post Doctoral Program of Rural Development Administration, National Livestock Research Institute, Suwon, Korea (RDA-NLRI)– Philippine Council for Agriculture, Forestry and Natural Resources Research and Development (PCARRD) thru the Central Luzon State University in the Science City of Munoz, Nueva Ecija, Philippines.
(adsbygoogle = window.adsbygoogle || []).push({});
References
- American Conference of Governmental Industrial Hygienists (ACGIH). n- Valeraldehyde. In: TLVs®and other occupational exposure values – 1999. [CD- ROM]. Cincinnati OH, USA.
- American Public Health Association, American Water Works Association, and Water Environment Federa 1998: Standard methods for the examination of water and wastewater. 20th ed. APHA, Washington, DC.
- Amoore, J.E. and E. Hautala .1983. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl Toxicol. 3: 272-90.
- Cho, J.H., Y.J. Chen, B.J. Min, H.J. Kim, S. Kwon, K.S. Shon, I.H. Kim, S.J. Kim and A.:80-85. Asamer. 2006. Effects of essential oils supplementation on growth performance, IgG concentration and fecal noxious gas concentration of weaned pigs. Asian-Aust. J. Anim. Sci. 19:80-85
- Chen Y.J., O.S. Kwon, B.J. Min, K.S. Shon, J.H. Cho and I.H. 2005. The effects of dietary Biotite V supplementation on growth performance, nutrient digestibility and fecal noxious gas content in finishing pigs. Asian-Aust. J. Anim. Sci. 18: 1147 – 1152.
- Chen, Y.J., B.J. Min, J. Cho, O.S. Kwon, K.S. Son, I.H. Kim and S.J. Kim. 2006a. Effects of dietary Enteroccus faecium SF68 on growth performance, nutrient digestibility, blood characteristics and faecal noxious gas content in finishing pigs. Asian-Aust. J. Anim. Sci. 19: 406-411.
- Chen, Y.J., B.J. Min, J. Cho, O.S. Kwon, K.S. Son, I.H. Kim and S.J. Kim. 2006b. Effects of dietary Bacillus-based probiotic on growth performance, nutrient digestibility, blood characteristics and fecal noxious gas content in finishing pigs. Asian-Aust. J. Anim. Sci. 19: 587-592.
- Claus, R., Lősel, M. Lacorn, J. Mentschel and H. Schenkel. 2003. Effect of butyrate on apoptosis in the pig colon and its consequences for skatole formation and tissue accumulation. J. Anim. Sci. 81:239-248.
- CUOEL. Health-based Reassessment of Administrative Occupational Exposure Limits. Committee on Updating of Occupational Exposure Limits of the Health Council of the the Hague, Netherlands.
- Eduard, W., P. Sandven and F. Levy. Serum antibodies to mold spores in two Norwegian sawmill populations: relationship to respiratory and other work- related symptoms. Am. J. of Industr. Medicine. 24:207-222.
- Environment Canada and Health Canada, 19 Canadian Environmental Protection Act Priority Substances List Supporting Document: Xylenes – Unedited Document.
- Fra E.W., L.A. Restaino, L. Blaszko. 1988. Evaluation of β-glucuronidase substrate 5-bromo-4-chloro-3-indol-β-D-glucuronide (X-GLUC) in a 24 hour direct plating method for Escherichia coli.-J. Food Protection, 51; 402-404
- Goldstein, 2002. Principles and Practice. 2002. Quick-to-implement odor reduction techniques. BioCycle. January. pp. 29-30.
- Hamilton, W., and H.J. Cumba.2000. Thermal phenomena in animal waste treatment lagoons. In Animal, agricultural and food processing wastes. Proceeding of the 8th Int. Symp. Am. Soc. of Agric. Eng., Sr. Joseph, MI. p. 672-678.
- Hobbs, P.J., B.F. Pain, R.M. Kay, and P.A. Lee. 1 Reduction of odorous compounds in fresh pig slurry by dietary control of crude protein. J. Sc. Food Agric. 71:508-514.
- Holloway, P. (2002). Odor-generating bacteria in swine manure and composted swine manure. Final Report submitted to Manitoba Livestock Manure Management Initiative. 6 pp.
- Hovers T. Protein in pre-starter diets, an unavoidable evil. Asian Pork. June/July pp. 18-21.
- Hurley, J.C. Endotoxemia: methods of detection and clinical correlates. Clin. Microbiol. Rev. 8:268-92.
- Jacobson, L., J. Lorimor, J. Bicudo and D Sc 2001. Emission control strategies for building sources. In USDA/EPA National Manure Stewardship Curriculum. Lesson 41.
- JEQ Executive Summaries (2005). This issue in Journal of Environment and Quality. Hard Copy. Published online. Nov. 7, 2005. 6 pp.
- Johansson, B., B. Drake and B. Berggre 1973. Detection thresholds: effect of stimulus presentation order and addition of blanks. I. Odor of pentanal and hexanol. Lebensm-Wiss Technol. 6: 115-22.
- Kempen, T.V. and E.V. Heugte 2005. Impact of diet on odor. Annual Swine Report. College of Agriculture and Life Sciences. North Carolina State University.
- Kilian, M. A., P. Bülow. 1976. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidase Acta Pathol. Microbiol. Scand. Sect. B 84; 245-251
- Mackie, R.I., PG. Stroot and V.H, Vare 1998. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 76:1331-1342.
- Malmros, P., T. Sigsgaaard and B. Bac 1992. Occupational health problems due to garbage sorting. Waste Management and Research. 10:227-234.
- Manafi, M. A., W Kneife(1989) A combined chromogenic-fluorogenic medium for the simultaneous detection of total coliforms and E. coli in water. Zentralabl. Hyg.189; 225-234.
- Martin, W. T., Y. Zhang, P. Willson, T.P. Archer, C. Kinahan and Barber. 2005.Bacterial and fungal flora of dust deposits in a pig building. http://www.age.uiuc.edu/bee/RESEARCH/Dust/Dust1.htm . Retrieved June 15, 2005.
- Merck Index. 1968. An Encyclopedia of Chemicals and Drug 8th Ed., Merck & Co., Inc., Pahway, NJ.
- Merril, L. and L.J. Halver 2002. Seasonal variation in microbial communities and organic malodor indicator compound concentrations in various types of swine manure storage systems. J. Environ. Qual. 31:2074–2085.
- Mitsuoka, T., Ohno, Y. Benno, K. Suzuki, and K. Nanba. 1976. Die Faekalflora bei Menshen. IV. Mitteilung : Vergleich des neu entwickelten Verfahrens mit dem bisherigen ublichen Verfahren zur Darmfloraanalyse. Zentrabl. Bakteriol. Parasitenkd. Infectionskr. Hyg., I. Abt. Orig. A 234:219-233.
- Murry, C., P. Bush and A. Hinton, Jr. 2002. Effect of lactosucrose on performance, fecal odorous compounds, and fecal microflora in growing pigs. The Univ. of Georgia, CAES, Dept. of Animal & Dairy Sci., 2001/2002 Annual report. 3 pp.
- National Research Counc 1998. Nutrient Requirements of Swine. 10th Ed. National Academy Press, Washington, DC.
- New Jersey Department of Health and Senior Services(NJDHSS). Hazardous substance. Fact Sheet.www.state.nj.us/health/eoh/rtkweb/1866.pdf#search=’Toluene’ retrieved January 23, 2006.
- Obrock, , C.E., P.S. Miller, A.J. Lewis, and R.K. Shoemaker. 1997. The effect of reducing dietary protein concentration on odor emission from pig buildings. Presented at American Society of Animal Science Midwestern Section, March 17-19, Des Moines, IA. Abstract number 38.
- 2001. Ontario air standards for xylene. Ontario Standard Bureau. Ontario Ministry of Environment. PDF format.
- Ohta, Y. and Ikeda (1978). Deodorization of pig feces by Actinomycetes. Appl. Environ. Microbio. 36:487-491.
- Otto, E.R., M. Yokoyama, S. Hengemuehle, R.D. von Bermuth, T. van Kempen and N.L.Trottier. Ammonia, volatile fatty acids, phenolics, and odor offensiveness in manure from growing pigs fed diets reduced in protein concentration. J. Anim. Sci. 81:1754 -1763
- Owasu-Asiedu, , C.M. Nyachoti and R.M. Marquardt. 2003. Response of early- weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J. Anim.Sci.. 81: 1790-1798.
- Ritter, W.F. Odor control of livestock wastes: State-of- the-art in North America. J.Agric. Eng. Res. 42:51-62.
- Rosenfield, P., Grey and M. Suffet. 2002. Controlling odors using high carbon wood ash. BioCycle. March. pp. 42-46.
- Salem, H. and Cullumbine, H. 1960. Toxic Appl. Pharmacol. 2, 183-187.
- SIDS. 2000. SIDS Initial Assessment Report for SIAM 10. UNEP Publica PDF format. 58 pp
- Simpson, J.M., B. Martin, W.E. Jones, J. Ballam and R.I. Mackie. 2002.
Characterization of fecal bacterial population in canines: Effects of age, breed and dietary fiber. Microbial Biology. 44:186-197.
- SPSS 00 Computer Software (1999) SPSS Inc, Headquarters, 233 s., Wacker Drive, Chicago, Illinois 60606.Standards Development Bureau(SDB). 2001.Ontario air standards for xylene. Ontario Ministry of the Environment. 60 pp.
- Sutton, A.L., J.A. Patterson, L. Adeaola, B.A. Richert, D.T. Kelly, A.J. Heber, K.B.Kephart, R. Mumma, and E. Bogus. 1998. Reducing sulfur-containing odors through diet manipulation. In: Proceedings of the Animal production Systems and the Environment: An International Conference on Odor, Water Quality, Nutrient Management and Socioeconomic Issues. Des Moines, IA, July 19-22, pp. 125-130.
- Sutton, A.L. K.B. Kebhart,M.W.A. Verstegen, T.T. Canh, and P.J. 1999. Potential for reduction of odorous compounds in swine manure through diet modification. J. Anim.Sci. 77:430-439.
- Terada, A., H. Hara, T. Oishi, S. Matsui, T. Mitsuoka. S. Nakajyo, I. Fujimori and K.Hara. 1992. Effect of dietary lactosucrose on faecal flora and faecal metabolites of dog Microbial Ecol. Health Dis., 5: 87-92.
- Van Heugten, , D.W. Funderburke and K.L. Dorton. 2003. Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J. Anim. Sci. 81:1004-1012.
- Van Kempen, T and Van Heugten. 2003. Impact of diet on odor. Annual Swine Report. North Carolina State University.10 pp.
- Whitehead, T.R. and A. Cotta.2001. Characterization and comparison of microbial populations in swine faeces and manure storage pits by 16S r DNA gene sequence analyses. Anaerobe. 181-187.
- Whittington, L. and S. Lemay. 2006. Greenhouse gases and odor contr http://www.prairieswine.usask.ca/whatsnew/November2004/GHG.pdf Date retrieved January 27, 2006.
- Xu, C., Chen, C. Ji, Q. Ma, and K. Hao. 2005. Study of the application of fructooligosachharides in piglets. Asian-Aust. J. Anim. Sci. 18:1011-1016
- Yen, J.T., V.H. Varel and J.A. Nienaber. Metabolic and microbial responses in western crossbred and Meishan growing pigs fed a high fiber diet. J. Anim.Sci..82:1740-1755.
- Yang, C.B., Y.H. Yoo, J. Barroga, T.I. Kim and J.I. Song. 2005. Reduction technologies development for odor from livestock production in Korea. In : Proceeding of International Symposium on Varying Global Strategies for Greenhouse Gas and Odor Reduction in Livestock. Jeju, Korea. pp. 71-93.
- Zhu, J., and LD Jac 1999. Correlating microbes to major odorous compounds in swine manure. Quali. 29: 737-744.
Table 1: The formulated diets for growers and finishers and their chemical composition
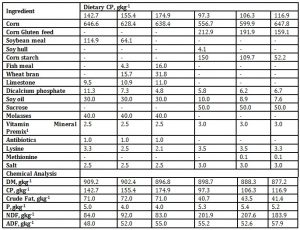
1 Provided the following kilogram of diet: vitamin A 5,500 IU. Vitamin E 27 IU, menadione sodium bisulfate 2.5mg, panthotenic acid 27 mg, niacin 33mg, riboflavin 5.5mg, vitamin B12 0.04mg, thiamin 5mg, pyridoxine 3 mg, biotin 0.24 mg, folic acid 1.5 mg, choline chloride 700mg, selenium 0.15 mg, manganese 0.03 g, zinc 0.1 g, iron 0.1 g, iodine 0.5 mg, magnesium 0.1g
Table 2: Incubation conditions for microbial enumeration
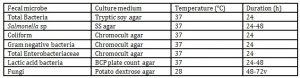
Table 3: Influence of DPL and season on fecal microbe shedding in growing pigs, (Unit: CFUg-1)

Mean weight of all pigs during mid spring, late spring and early summer collection were 25kg, 32 kg and 40 kg, respectively
*means within a row followed by the same letter are not significantly different according to DMRT (p<.05)
** Colony Forming Unit
Table 4: Influence of DPL and season on fecal microbe shedding in finishing pigs (Unit: CFUg-1)

Table 5: Effect of DPL (bodyweight) and season on iso-valeraldehyde emission (Unit: mgL-1)
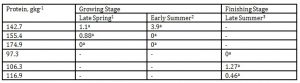
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 Body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 6: Effect of DPL (bodyweight) and season on valeraldehyde emission (Unit: mgL-1)
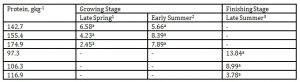
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 Body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 7: Effect of DPL (bodyweight) and season on methyl isobutyl emission (Unit: mgL-1)
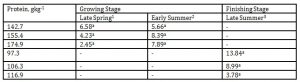
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 8: Effect of DPL (bodyweight) and season on butanol emission (Unit: mgL-1)
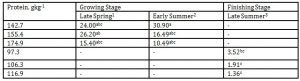
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 9: Effect of DPL (bodyweight) and season on butyric acid emission (Unit: mgL-1)
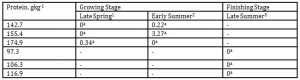
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 10: Effect of DPL (bodyweight) and season on xylene emission (Unit: mgL-1)
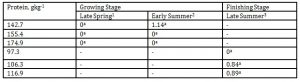
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)
Table 11: Effect of DPL (bodyweight) and season on toluene emission (Unit: mgL-1)
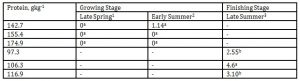
1 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 27.68, 30.98 and 33.28 kg, respectively
2 Body weights of pigs offered 142.7, 155.4 and 174.9 gkg-1 CP are 35.55, 38.34 and 40.79 kg, respectively
3 body weights of pigs offered 97.3, 106.3 and 116.9 gkg-1 CP are 51.81, 55.98 and 68.39 kg, respectively
*Means within a column followed by the same letter are not significantly different according to DMRT (p<0.05)














