Introduction
Osteoarthritis (OA) is a highly prevalent disease and the prevalence increases with age as a result of urbanization and increased longevity. Hayes et al (2005) found that the OA- related complaints are very common. Jimenez et al (1995) reported that the osteoarthritis (OA) is a progressively debilitating disease that affects mostly cartilage, with associated changes in the bone. The lack of therapeutic choices has led to focus on the potential of platelet rich plasma as a new strategy for cartilage repair. In this study, the scaffold free autogenous platelet rich plasma (PRP) in an experimental animal model of OA by direct intra articular injection was used.
Materials and Methods
Preparation of PRP: Four milliliter blood was collected from the jugular vein of each rabbit into a sodium citrate tube. The tubes were centrifuged at 1240 rpm for eight minutes. The tubes showed three different density compartment, the lower red blood cells, the middle buffy coat of white blood cells, and the top plasma. The plasma had three distinct layers in ratio of 2:1:1 from the top. The first top layer was platelet poor plasma (PPP), the middle plasma average platelet (PAP) and the lower platelet rich plasma (PRP). The first (PPP) and the second (PAP) were removed by pipette. The third (PRP) layer was carefully separated by pipette and centrifuged again for 5 minutes at the same speed. Then the first layer (plasma) was discarded and the second layer (PRP) was collected for intra articular injection in the OA joints.
Surgical procedure: The study was approved by institutional ethical committee. Seventeen adult New Zealand white rabbits weighing 2.22±0.12 kg were used. The rabbits were anesthetized by intramuscular administration of 10 mg/kg of Xylazine (Rompun, Bayer AG, Leverkusen) and 50 mg/kg of ketamine (Alfasan, Woerden-Holand). The knee was placed in full flexion and under aseptic conditions through the skin incision in the medial para-patellar area of the left knee the patella was displaced laterally and the anterior cruciate ligament was transected (ACLT). The joint capsule and subcutaneous tissue were closed using 3-0 Polydioxanone suture (ETHICON, INC). The skin was closed using silk suture (SUPA, Iran). Following surgery, rabbits were treated by a standard antibiotic (Penicillin, Zakaria laboratory Tabriz, Iran), and analgesic (Flunixin, Razak laboratories, Tehran-Iran). They were allowed to resume normal cage activity for 8 weeks.
PRP injection: Ten out of 17 rabbits were anesthetized at the end of 8 weeks following surgery. The knee joint was prepared for aseptic injection. 0.5 ml PRP was injected into the medial compartment of the operated joint. The remaining 7 rabbits served as the control group and received no injection. All the rabbits were allowed unrestricted cage activity without immobilization until sacrifice.
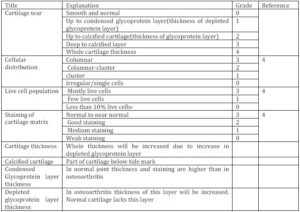
Pathologic evaluation: At the 12th week after the initial surgery five rabbits from the treatment group and three rabbits from the control group were euthanized using sodium thiopental overdose. At 16th week post surgery, the remaining rabbits in treatment group and control group were also euthanized. The knee tissues were fixed into 10% buffered formalin. Then the specimens were kept in 7% nitric acid for 48 to 72 hours to allow the decalcification process. Samples were cut in frontal plan, so that femur and tibia and joint space in between could be seen in a single plane. Samples were stained by toluidine blue solution (2%). Thickness of cartilage, chondrocyte count, presence and severity of cartilage tear(s), glycoprotein content, collagen staining quality and assessment of the proportion of live chondrocytes were determined for quantitative measures. Thickness of the cartilage was measured using a scaled graticule lens at 10X objective and multiplication by 10.82. This measurement was done on both femoral and tibial sides of the joint space in five randomly selected areas. To determine the quantity of chondrocytes, a reticulated graticule lens was used to read cells in five different, random areas. The presence and severity of cartilage tears was graded by the following criteria:
Grade 0: no tears
Grade 1: tear in depleted glycoprotein layer
Grade 2: tear up to calcified layer
Grade 3: tear in calcified cartilage up to the underlying bone
Grade 4: tear extending in to the bone
Glycoprotein content was measured by using tide mark level of cartilage (boundary between glycoprotein layer and calcified cartilage) as a guideline. The collagen staining quality was assessed by toluidine blue. Assessment of cartilage cell distribution was performed by evaluating pattern of distribution as: columnar, columnar and cluster, cluster. Live chondrocyte proportion was measured by counting cartilage lacunae that contained visible chondrocytes with intact nucleus and cell membrane in five different randomly chosen areas.
Table 1: Measured Histological Attributes and Their Grading System
Statistical Analysis:
The data (mean± SE) were analyzed statistically by SPSS version 18, using paired t- test, Anova and Tukey test. The values less than 0.05 were considered as significant.
Results
Results of measured data in treatment and control groups at 12th and 16th week are summarized in tables 2 and 3 and in histograms 1 and 2.
Table 2: The Values are Presented in Mean ± SE for Four Groups
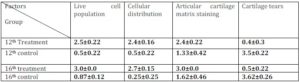
There was no significant (P>0.05) difference in the thickness of condensed glycoprotein layer and the thickness of calcified cartilage between the control and treatment groups at 12th and 16th weeks and in total cartilage thickness at 12th week. There was a significant (P<0.05) difference between the thickness of depleted glycoprotein layer at 12th and 16th week and in total cartilage thickness (P<0.05) at 16th week (Table 3)
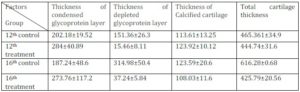
Table 3: The Values are Presented in Mean ± SE for Four Groups
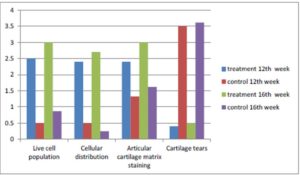
Histogram 1: Mean of measured histological data at 12th and 16th week. Significant (P<0.05) difference in all measured data were observed.
There was no significant (P>0.05) difference in the thickness of condensed glycoprotein layer and calcified cartilage between control and treatment groups at 12th and 16th week as well as in total cartilage thickness in control and treatment group at 12th week.
There was significant (P<0.05) difference between the thickness of depleted glycoprotein layer at 12th and 16th week as well as in total cartilage thickness at 16th week (histogram 2).
Histogram 2: Mean of measured histological data at 12th and 16th week (μm).
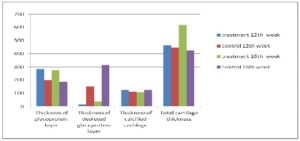
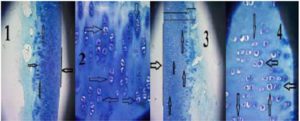
Fig.1. Treatment Group at 12th Week. (1): Smooth Articular Surface (Vertical Line), Columnar Distribution of Cells (Arrow), (2): Live Chondrocytes with Columnar Distribution and Fair Staining. (3): Smooth Surface (Vertical Line), Columnar Distribution of Cells (Arrows), Whole Thickness of Cartilage (Long Horizontal Line), Condensed Glycoprotein Layer (Medium Horizontal Line), Calcified Cartilage (Short Horizontal Line). (4): Arrows Pointing to Live Chondrocytes and Their Pattern of Distribution. Toluidine Blue Staining. (1&3) x10, (2&4) x40

Fig. 2. Control Group at 12th Week. (5) Inner Surface of Cartilage (Horizontal Lines), Cartilage Tears (Arrow), Total Cartilage Thickness (Long Vertical Line), Cartilage Tears (Horizontal Arrows), Cartilage Devoid of Live Cells (Vertical Arrow). (6) Total Cartilage Thickness (Vertical Line), Cluster Distribution of Cells (Circle). (7) Rough Cartilage Surface (Arrow), Fibrosis of Cartilage and Absence of Live Cells (Circle), Remaining Live Chondrocytes with Cluster Distribution (Ellipse). (8) Cartilage Tear (Arrows), Difference in Cartilage Thickness (Circles). Toluidine Blue Staining. (5&6) x10, (7) x40, (8) x4

Fig. 3. Treatment Group at 16th Week. (1) Smooth Cartilage Surface (Horizontal Line), Columnar Distribution of Live Chondrocytes and Good Staining of Cartilage (Arrows). Smooth Cartilage Surface (Horizontal Line), Columnar Cellular Distribution (Arrows and Circle), Whole Thickness of Cartilage (Long Vertical Line), Calcified Cartilage (Short Vertical Line). Toluidine Blue Staining. (1&2) x10, (3&4) x40
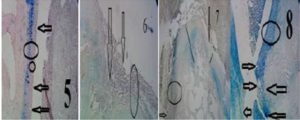
Fig. 4. Control Group 16th at Week. (5) Cartilage Tears (Arrows), Total Cartilage Thickness (Large Circle), and Depleted Glycoprotein Layer which Makes Most of Cartilage Thickness (Small Circle). (6) Cartilage Tear up to the Underlying Bone (Arrows), Cartilage Thickness Composed of Totally Fibrous Tissue (Circle). (7) Cartilage Tears (Arrows), Different Cartilage Thickness (Vertical Lines), and Replacement of Cartilage by Fibrous Tissue). (8) Cartilage Tears (Arrows), Total Cartilage Thickness (Circle). Toluidine Blue Staining. (5, 7&8) x4, (6) x10
Discussion
Osteoarthritis is one of the most debilitating joint diseases and has major influences on the life style, and characterized by cartilage deterioration, loss of joint space, osteophytes and loss of joint function. Griffine et al, (2009) mentioned that PRP contains various growth factors that play important roles in cell proliferation, chemotaxis, cell differentiation, and angiogenesis concentrations. The basic cytokines identified in PRP include platelet-derived growth factor, transforming growth factor β, vascular endothelial growth factor, hepatocyte growth factor, fibroblast growth factor, epidermal growth factor, and endothelial cell growth factor. Xiaofeng et al, (2011) mentioned that the clinical benefit of these growth factors contained in PRP or BMC (bone marrow concentrate) for the treatment of pathologies of the foot or ankle is derived from these biological properties. Gruber et al, (2002) said that platelets and thrombin activated platelet products induced mitogenic activity of cultured human trabecular bone-derived cells and that platelet concentrates also enhance the proliferation of human osteoblast like cells. PRP would increase demineralized bone matrix osteoinductivity in vivo. Chondrogenesis has been observed in 2 weeks and osteogenesis in 4 weeks in the non activated PRP cohort.
Han et al, (2009) reported that thrombin activation would yield inflammatory cells that were not seen in the non activated group. Hurst and Bazan, (1995) reported that the platelet-activating factor (PAF) produces several biochemical responses associated with inflammation and wound healing. Bazan and Tao, (1997) mentioned that it also activates mitogen-activated protein kinases and stimulates both calcium influx into cells and the expression of the cyclooxygenease (COX-2) enzyme, an inducible isoform responsible for synthesis of various prostaglandins associated with inflammation. In a research by Bazan et al, (1993), Tao et al, (1995) and Tao et al, (1996) the PAF activates the gene expression of selective metalloproteinases (MMPs) involved in tissue remodeling, such as MMP-1 and MMP-9, urokinase plasminogen activator (uPA), and their inhibitors.
This study described process of development of Osteoarthritis (OA) in rabbits by pathological investigation and also the effects of platelet rich plasma on repair process and conversion of osteoarthritis changes to normal articular structure. Bone alterations in an advanced OA include subchondral cysts and formation of osteophyte. However, Findlay (2007) believed that the lack of understanding of the underlying cause(s) for OA mean that treatments remain largely palliative. Goldring and Goldring (2006) mentioned that as OA progresses, there is evidence of vascular invasion and advancement of this zone of calcified cartilage into the articular cartilage that further contributes to a decrease in articular cartilage thickness. Platelet-derived Growth Factors (GFs) enhance tissue repair mechanisms such as angiogenesis, extracellular matrix remodeling, and cellular effects as stem cells recruitment, chemotaxis, cell proliferation and differentiation due to their biologically active peptides. Platelet growth factors have proved a certain therapeutic modality, offering opportunities for treatment of soft-tissue injuries, cutaneous wounds, ulcers, and more in cell therapy. Ahmad et al, (2012) reported that the Platelet rich plasma (PRP) is an autologous blood-derived product that has an increased concentration of platelets that are rich in growth factors, and has the potential to enhance the healing of tissue at the cellular level via the recruitment, proliferation, and differentiation of cells involved in tissue regeneration Gotterbarm et al, (2006) said the platelet-rich plasma (PRP) acts as a rich source of autologous growth factors. Thus Sun et al, (2010) mentioned that, PRP is a very good clinical source for osteochondral regeneration. Shin et al, (2012) proved that PRP was effective in normal tissue regeneration. But there is no clue that platelet rich plasma would normalize the diabetic wound healing pathway. In a study Madlener et al, (1998) observed an extracellular matrix-regulating effect of PRP. In this study the beneficial effects of PRP were observed in the OA joints.
(adsbygoogle = window.adsbygoogle || []).push({});
References
Ahmad, Z., Howard, D., Brooks, R. A., Wardale, J., Henson, F. M. D., Getgood, A. & Rushtun, N. (2012). “The Role of Platelet Rich Plasma in Musculoskeletal Science,” Journal of the Royal Society of Medicine, 3(6) 40-45.
Publisher – Google Scholar
Bazan, H. E. P., Tao, Y. & Bazan, N. G. (1993). “Platelet-Activating Factor Induces Collagenase Expression in Corneal Epithelial Cells,” Proceedings of the National Academy of Sciences of the United States of America. 90, 8678–8682.
Publisher – Google Scholar
Bazan, H. E. P., Tao, Y., DeCoster, M. A. & Bazan, N.G. (1997). “Platelet Activating Factor Induces Cyclooxygenase-2 Gene Expression in Corneal Epithelium. Requirement of Calcium in the Signal Transduction Pathway,” Investigation Ophthalmological & Visual Science. 38, 2492–2501.
Publisher – Google Scholar
Bazan, H. E. P. & Varner, L. (1997). “A Mitogen-Activated Protein Kinase (MAP-Kinase) cascade is Stimulated by Platelet Activating Factor (PAF) in Corneal Epithelium,” Current Eye Research, 16, 372– 379.
Publisher – Google Scholar
Chen, W.- H., Lo, W.- C., Lee, J.- J., et al. (2006). “Tissue-Engineered Intervertebral Disc And Chondrogenesis Using Human Nucleus Pulposus Regulated through TGFbeta1 in Platelet-Rich Plasma,” Journal of Cellular Physiology. 209:744-754.
Publisher – Google Scholar
Findlay, D. M. (2007). “Vascular Pathology and Osteoarthritis,” Journal of Rheumatology. 46: 1763-1768.
Publisher – Google Scholar
Goldring, S. R. & Goldring, M. B. (2006). “Clinical Aspects, Pathology, and Pathophysiology of Osteoartheritis,” Journal of Musculoskeletal Neuronal Interact. 6 (4): 376- 378.
Publisher – Google Scholar
Gotterbarm, T., Richter, W., Jung, M., Berardi, S., et al. (2006). “An in Vivo Study of a Growth-Factor Enhanced, Cell Free, Two-Layered Collagen-Tricalcium Phosphate In Deep Osteochondral Defects,” Biomaterials, 27:3387–3395.
Publisher – Google Scholar
Griffin, X. L., Smith, C. M. & Costa, M. L. (2009). “The Clinical Use of Platelet-Rich Plasma in the Promotion of Bone Healing: A Systematic Review,” Injury International Journal of the Care of the Injured. 40, 158–162.
Publisher – Google Scholar
Gruber, R., Varga, F., Fischer, M. B. & Watzek, G. (2002). “Platelets Stimulates Proliferation of Bone Cells: Involvement of Platelet-Derived Growth Factor, Microparticles and Membranes,” Clinical Oral Implants Research.13:529-535.
Publisher – Google Scholar
Han, B., Woodell-May, J., Ponticiello, M., Yang, Z. & Nimni, M. (2009). “The Effect of Thrombin Activation of Platelet-Rich Plasma on Demineralized Bone Matrix Osteoinductivity,” Journal of Bone and Joint Surgery American. 91:1459-1470.
Publisher – Google Scholar
Hayes, C. W., Jamadar, D. A., Welch, G. W., Jannausch, M. L., Lachance, L. L., Capul, D. C. & Sowers, M. F. R. (2005). “Osteoarthritis of the Knee: Comparison of MR Imaging Findings with Radiographic Severity Measurements and Pain in Middle-Aged Women,” Radiology, 237(3): 998-1007.
Publisher – Google Scholar
Hurst, J. S. & Bazan, H. E. P. (1995). “Activation of the Phospholipase/ Cyclooxygenase Cascade in the Rabbit Cornea by Platelet-Activating Factor is Challenged by PAF receptor Antagonists,” Journal of Ocular Pharmacology and Therapeotics. 11, 329–337.
Publisher – Google Scholar
Jia, X., Peters, P. G. & Schon, L. (2011). “The Use of Platelet-Rich Plasma in the Management of Foot and Ankle Conditions,” Operative Techniques in Sports Medicine. 19:177-184.
Publisher – Google Scholar
Jimenez, P. A., Harlan, P. M., Chavarria, A. E. & Haimes, H. B. (1995). “Induction of Osteoarthritis in Guinea Pigs by Transection of the Anterior Cruciate Ligament: Radiographic and Histopathological Changes,” Inflammation Research. 44 (Suppl 2):S129–130.
Publisher – Google Scholar
Madlener, M., Parks, W. C. & Werner, S. (1998). “Matrix Metalloproteinases (MMPs) and Their Physiological Inhibitors (TIMPs) are Differentially Expressed during Excisional Skin Wound Repair,” Experimental Cell Research. 242:201–210.
Publisher – Google Scholar
Shin, H. S. & Oh. H. Y. (2012). “The Effect of Platelet Rich Plasma on Wounds of OLETF Rats Using Expression of Matrix Metalloproteinase-2 and -9 mRNA,” Archives of Plastic Surgery. 39(2): 106–112.
Publisher – Google Scholar
Sun, Y., Feng, Y., Zhang, C.Q., Chen, S. B. & Cheng, X. G. (2010). “The Regenerative Effect of Platelet-Rich Plasma on Healing in Large Osteochondral Defects,” International Orthopedics. 34(4): 589–597.
Publisher – Google Scholar
Tao, Y., Bazan, H. E. P. & Bazan, N. G. (1995). “Platelet-Activating Factor Induces the Expression of Metalloproteinases-1 and -9, but not -2 or -3, in the Corneal Epithelium,” Investigation Ophthalmology & Visual Science. 36, 345–354.
Publisher – Google Scholar
Tao, Y., Bazan, H. E. P. & Bazan, N. G. (1996). “Platelet-Activating Factor Enhances Urokinase-Type Plasminogen Activator Gene Expression in Corneal Epithelium,” Investigation Ophthalmology & Visual Science. 37, 2037–2046.
Publisher – Google Scholar











