Introduction
Fractures that result either from osteoporosis or coronary artery disease (CAD) caused a decrease in the quality of life, increases in health care burdens and considerable morbidity and mortality.
Growing evidence suggests significant associations between these two diseases. Patients with osteoporosis or osteopenia have a higher prevalence of obstructive coronary artery disease, atherosclerotic vascular disease, and stress test-induced myocardial ischemia than those with normal bone mineral density (BMD) (Varma et L,2008; Pierre-Louis et al., 2009; Gupta and Aronow, 2006). Meanwhile, BMD is a more significant predictor of cardiovascular death in white men and CAD in women than traditional cardiovascular risk factors (Marcovitz et al., 2005; Mussolino et al., 2003). Furthermore, calcium supplements with or without vitamin D used for the prevention and treatment of osteoporosis modestly increase the risk of cardiovascular events, especially myocardial infarction (Bolland et al., 2011). In addition, the 10-year Framingham Coronary Heart Disease Risk Assessment is useful for women to identify osteoporosis (Broussard and Magnus, 2008). Also, there is evidence that drugs given to treat cardiovascular disease may impact bone health in either beneficial or harmful ways (Walsh et al., 2012). However, other studies proved that neither cardiovascular risk factors nor CAD were relevant to low BMD in either men or women (Mussolino and Armenian, 2007; Tekin et al., 2008; Broussard and Magnus, 2008).
A reasonable explanation to this contradictory phenomenon is that both CAD and osteoporosis are age dependent (Yetkin et al., 2008), therefore, it is necessary to evaluate these two diseases at different ages. We evaluated the influence of cardiovascular risk factors including smoking, hypertension, diabetes mellitus and hyperlipidemia on osteoporosis among CAD patients of varying ages.
Methods
Study Population
The study population consisted of consecutive patients in the cardiovascular department of Shanghai First People’s Hospital during the period from May 2012 to October 2012, excluding ones using steroids, estrogens or calcium treatment, or with hepatic failure, renal failure, acute inflammatory, malignant tumors or hyperparathyroidism. All of them were born in Shanghai, China. Informed consent was obtained from each subject, and the study was approved by the ethics committee of Shanghai First People’s Hospital.
Clinical Characteristics
Patients were asked to complete a questionnaire about the history of hypertension, diabetes, hyperlipidemia and smoking, the family history of CAD and the use of antihypertensive, hypoglycemic drugs and antihyperlipidemics. Smoking was defined as active smoking within the previous one year. The patients weighed in light clothing (within 0.5kg), without shoes. Height deviation was controlled to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Blood pressure was taken using a mercury-gravity sphygmomanometer in a sitting position after a 5-min rest.
Biochemical Parameters
After a 12-h overnight fast, blood samples were collected from study population, and total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), apolipoprotein E (ApoE), apolipoprotein A-I (ApoA-I), apolipoprotein B (ApoB), lipoprotein(a) (Lp(a)), glycosylated hemoglobin (HbA1c), Glycated albumin and fasting plasma glucose (FPG) were tested. Two hours after breakfast, blood samples were gathered again, and 2h plasmaglucose (2hPG) was measured. All of these blood samples were tested within 30 min at the clinical laboratory in the Shanghai First People’s Hospital.
Coronary Angiography
Coronary angiography was performed with the standard Judkin’s technique by experienced cardiologists for evaluation of known or suspected CAD among all patients. Those cardiologists knew nothing about the results of BMD. Normal luminal diameter of coronary artery was counted for 100%. If luminal narrowing >50%, at least in a main branch of the coronary artery, the patients were diagnosed as CAD. Meanwhile, patients undergoing previous percutaneous coronary intervention or coronary artery bypass grafting also belonged to CAD patients.
BMD Measurement
BMD was measured at the lumbar spine (L1 to L4) and total femur by a dual-energy x-ray absorptiometry (DEXA) scanning at the nuclear medicine department during the hospital stay. The T-score was calculated as bone mineral content corresponding to the number of standard deviations from the mean of a gender-matched and race-matched reference population of young adults(20~40 years old), as provided by the manufacturer. Osteoporosis was defined according to lowest measured T-score value in either spine or femur. BMD results were classified according to the World Health Organization criteria as normal (T score >-1.0 SD), osteopenia (T score -1.0 to -2.5 SD), and osteoporosis (T score
Statistics
The SPSS statistical software package version 19.0 was used for all statistical analyses. The chi-square statistical test was used for all categorical variables; one-way analysis of variance (ANOVA) was used for all continuous variables. The data of univariate analysis was expressed as means ± SD or percentage. A step-down multivariate logistic regression analysis was used to determine the relationships between osteoporosis and various clinical variables. To find out predictors of osteoporosis, we estimated the odds ratio (OR) and associated 95% confidence interval (CI). In the logistic regression analysis, variable selection was finished when no candidate variables for entry were significant at p<0.05, and all those selected for entry remained significant at p
Results
Our database included 436 patients. However, the ones did not undergo DEXA scans, or were not diagnosed as CAD, or with incomplete information were excluded in the final statistical analysis. Thus, our data included 186 patients (129 men and 57 women).
Age as A Risk Factor for Osteoporosis
Baseline characteristics of patients were presented in Table 1. Compared with non-osteoporosis patients, osteoporosis patients showed higher proportion in females, hypertension and family history of CAD and higher levels of age, HDL-C and apoA-I. Results of the step-down multivariate logistic regression analysis were presented in table 2. After adjustment, four risk factors were significantly associated with the presence of osteoporosis: family history of CAD, HDL-C, age and hypertension, among which HDL-C (OR: 7.146, CI: 1.717-29.741) conferred the highest risk factor for significant osteoporosis.
Table 1: Comparison of clinic data in patients with and without osteoporosis
BMI=Body mass index; FPG=fasting plasma glucose; HbA1c=glycosylated hemoglobin; 2hPG=2h plasmaglucose; TC=Total cholesterol; TG= triglyceride; HDL-c=high density lipoprotein cholesterol; LDL-c=low density lipoprotein cholesterol; ApoE =apolipoprotein E, ApoA-I=apolipoprotein A-I, ApoB= apolipoprotein B; Lp(a)= lipoprotein(a); CAD= coronary artery disease.
Table 2: Logistic regression analysis for osteoporosis
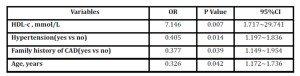
HDL-C=high density lipoprotein cholesterol; CAD=coronary artery disease; OR= odds ratio; CI=confidence interval.
Table 3: Logistic regression analysis for osteoporosis in the group aged above 65
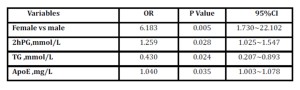
2hPG=2h plasmaglucose; TG=triglyceride; ApoE=apolipoprotein E; OR= odds ratio; CI=confidence interval
Table 4: Logistic regression analysis for osteoporosis in the group aged below 65
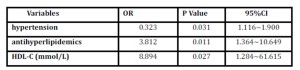
HDL-C= high density lipoprotein cholesterol; OR= odds ratio; CI=confidence interval
Risk Factor for Osteoporosis in Different age patients
In order to eliminate the effect of age on osteoporosis, we studied the relationship between cardiovascular risk factors and osteoporosis among patients of different ages, by dividing them into the elderly (>=65 years old) and the nonelderly (
For patients above 65, on univariate analysis, the different characteristics of patients who had osteoporosis and those who did not were HDL-C (1.21 vs 1.06, p=0.015), female gender (54.1% vs 17.9%, p= 0.001) and hyperlipidemia (48.6% vs 71.8%, p=0.039). However, age (74.68 vs 73.41, p=0.395) and BMI (24.76 vs 25.08, p=0.788) were not comparable. There were no significant differences between those with significant osteoporosis or not as to systolic pressure (135.00 and 136.51, respectively, p=0.727), diastolic pressure (75.03 vs 76.74, p=0.468), FPG (5.75 vs 5.30, p=0.261), HbA1c (6.61 vs 6.18, p=0.244), glycated albumin (16.01 vs 14.90, p=0.297) and 2hPG (9.48 and 8.69, respectively, p=0.395). In the osteoporosis group, 12.8% patients had family history of CAD, while in the non-osteoporosis group the number was 8.3% (p=0.529). TC (4.25 vs 4.16, p=0.740), TG (2.02 vs 1.91, p=0.800), LDL-C (2.41 vs 2.37, p=0.841), ApoE(54.70 vs 48.73, p=0.297), ApoA-I(1.28 vs 1.19, p=0.056), ApoB (0.89 vs 0.90, p=0.809) and Lp(a) (310.92 vs 298.24, p=0.813) were similar. In addition, history of hypertension, antihypertensive, diabetes mellitus, hypoglycemic drugs, antihyperlipidemics, and smoking were unrelated to the presence of osteoporosis. After adjustment, TG showed as the protective factor for osteoporosis as presented in table 3. On the contrary, female gender was the highest risk factor for osteoporosis among patients above 65.
For patients below 65, significant differences were found between the 2 groups in HDL-C (1.21 vs 1.05, p=0.005), ApoA-I (1.26 vs 1.16, p=0.011), antihyperlipidemics (33.3% vs 16.2%, p=0.037) and family history of CAD (35.4% vs 17.1%, p=0.042). There was no significant difference in other clinical data (data not shown). However, as shown in table 4, after adjustment, hypertension, antihyperlipidemics and HDL-C were all risk factors for osteoporosis for patients under 65 years old. Among these factors, HDL-C was the highest risk factor (OR: 8.894).
Discussion
In this study, we evaluated the impact of cardiovascular risk factors on osteoporosis among CAD patients. Firstly, we proved that age was independently correlated with clinically significant osteoporosis. Secondly, we demonstrated that cardiovascular risk factors for osteoporosis were various in different age groups. Female gender, 2hPG, TG and ApoE were relative factors for osteoporosis among patients older than 65 years, while, in patients younger than 65 years old, hypertension, antihyperlipidemics and HDL-C were associated with osteoporosis.
This study confirms that lipids had a greater impact on osteoporosis for people below 65, while for people above 65; female gender was more influential to osteoporosis. The reason for that phenomenon might be that elderly women were more inclined to lose bone because of a decline in oestrogen. Moreover, antihyperlipidemics were found harmful to bone health among patients younger than 65, and this finding was confirmed by previous studies (Walsh et al., 2012).
The effect of cardiovascular risk factors on osteoporosis demonstrated in this study was supported by those previous studies. Vestergaard et al., (Vestergaard et al., 2009) proved that hypertension was a well established risk factor for fracture and treatment reduced this risk, meanwhile, Wiens et al., (Wiens et al., 2006) reported that the use of antihypertensive, which included thiazide diuretics, nonthiazide diuretics and beta-blockers, could reduce any fracture in a meta-analysis of fifty-four observational studies. Adami et al., (Adami et al., 2004) discovered that high BMD was significantly associated with lower HDL cholesterol and higher TG after adjustment in both men and women, what’s more, Ackert-Bicknell.(Ackert-Bicknell, 2012) provided sufficient evidences to conclude that BMD and HDL were genetically linked. In the Framingham osteoporosis study, total cholesterol levels in women and men from young adulthood to middle age years also did not appear to have long-term clinical implications for osteoporosis later in life (Samelson et al., 2004). A recent study demonstrated that elderly patients with type 2 diabetes mellitus were prone to develop osteoporosis because of the insufficiency of insulin, the decreased insulin sensitivity and diabetic nephropathy (Xia et al., 2012).
However, we could not find an association between BMI and osteoporosis and more interestingly no association between smoking and osteoporosis was found. A meta-analysis for fracture risk showed that low BMI conferred a risk of substantial importance for all fractures (De et al., 2005). Furthermore, it was proved that mean BMD at femoral sites were significantly higher in obese women suggesting obesity as a protective factor for postmenopausal osteoporosis (Albala et al., 1996). Recently, a study based on a Chinese cohort found that current smoking independently associated with increased risk of CVD, in women and men (Shen et al., 2012). One obvious reason for this lack of association could relate to recall bias with respect to smoking and unknown confounders as to both BMI and smoking. On the other hand, our small sample could also be another explanation.
Study Limitations
Our study had several limitations. First, the study population were patients with poor health status and prone to have those two diseases. Second, patients with CAD tended not to be physically active, which resulted in a lower BMD. Third, patients might have had a recall bias with respect to risk factors. In addition, the results may not apply to be extrapolated to other countries and regions, because our study population was born in Shanghai, China. Finally, a history of CAD, which might strengthen our results, was not investigated.
Perspectives
In recent years, a large number of common risk factors for CAD and osteoporosis have been examined in order to reduce morbidity of these 2 diseases and consequently improve patient care. We believe that risk stratification partly based on cardiovascular risk factors, which include smoking, hypertension, diabetes mellitus and hyperlipidemia, and age may improve osteoporosis of CAD patients by optimizing clinical control, treatment and focus on patient compliance.
Even though the association between cardiovascular risk factors and osteoporosis seems apparent, the cause of this association is still poorly understood and future studies need to further clarify the common mechanisms, especially in vitro studies, animal studies, and large human studies.
Conclusion
The impact of cardiovascular risk factors on osteoporosis differed among CAD patients of various ages. Therefore, CAD patients of different ages with osteoporosis should be treated with corresponding risk-factor modification to reduce morbidity and mortality of both 2 diseases.
Acknowledgments
We thank all subjects who participated in the study.
Conflicts of interest
The present study was supported by the Natural Science Foundation of Shanghai, China (No 09ZR1425200).
References
1. Ackert-Bicknell, C. L. (2012) “HDL cholesterol and bone mineral density: is there a genetic link?” Bone , 50(2):525-33.
Publisher – GoogleScholar
2. Adami, S, Braga, V. and Zamboni, M. (2004) “Relationship between lipids and bone mass in 2 cohorts of healthy women and men,” Calcif Tissue Int , 74(2):136-142.
Publisher – GoogleScholar
3. Albala, C, Yáñez, M. and Devoto, E. (1996) “Obesity as a protective factor for postmenopausal osteoporosis,” Int J Obes Relat Metab Disord, 20(11):1027-1032.
GoogleScholar
4. Bolland, M. J, Grey, A. and Avenell, A. (2011) “Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis,” BMJ, 342:d2040.
GoogleScholar
5. Broussard, D.L. and Magnus, J. H. (2008) “Coronary heart disease risk and bone mineral density among U.S. women and men,” J Womens Health (Larchmt) ,17(3):479-90.
Publisher – GoogleScholar
6. Broussard, D. L. and Magnus, J. H. (2008) “Influence of cardiovascular disease risk factors on the relationship between low bone mineral density and type 2 diabetes mellitus in a multiethnic US population of women and men: a cross-sectional study,” Gend Med ,5(3):229-38.
Publisher – GoogleScholar
7. De Laet, C, Kanis, J. A. and Odén, A. (2005) “Body mass index as a predictor of fracture risk: a meta-analysis,” Osteoporos Int ,16(11):1330-1338.
Publisher – GoogleScholar
8. Gupta, G. and Aronow, W. S. (2006) “Atherosclerotic vascular disease may be associated with osteoporosis or osteopenia in postmenopausal women: a preliminary study,” Arch Gerontol Geriatr ,43(2):285-288.
Publisher – GoogleScholar
9. Marcovitz, P. A, Tran, H. H. and Franklin, B. A. (2005) “Usefulness of bone mineral density to predict significant coronary artery disease,” Am J Cardiol ,96(8):1059-1063.
Publisher – GoogleScholar
10. Mussolino, M. E. and Armenian, H. K. (2007) “Low bone mineral density, coronary heart disease, and stroke mortality in men and women: the Third National Health and Nutrition Examination Survey,” Ann Epidemiol , 17(11):841-846.
Publisher – GoogleScholar
11. Mussolino, M. E, Madans, J. H. and Gillum, R. F. (2003) “Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study,” Ann Epidemiol ,13(10):692-697.
Publisher – GoogleScholar
12. Pierre-Louis, B, Aronow, W. S. and Yoon, J.H. (2009) “Relation of bone mineral density to stress test-induced myocardial ischemia,” Am J Cardiol, 104(2):199-201.
Publisher – GoogleScholar
13. Samelson, E. J, Cupples, L. A. and Hannan, M.T. (2004) “Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study,” Bone ,34(3):557-561.
Publisher – GoogleScholar
14. Shen, C, Deng, J. and Zhou, R. (2012) “Relation between bone mineral density, bone loss and the risk of cardiovascular disease in a Chinese cohort,” Am J Cardiol ,110(8):1138-42.
Publisher – GoogleScholar
15. Tekin, G. O, Kekilli, E. and Yagmur, J. (2008) “Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography,” Int J Cardiol, 131(1):66-9.
Publisher – GoogleScholar
16. Varma, R, Aronow, W. S. and Basis, Y. (2008) “Relation of bone mineral density to frequency of coronary heart disease,” Am J Cardiol , 101(8):1103-4.
Publisher – GoogleScholar
17. Vestergaard, P, Rejnmark, L. and Mosekilde, L. (2009) “Hypertension is a risk factor for fractures,” Calcif Tissue Int, 84(2):103-1.
GoogleScholar
18. Walsh, J. S, Newman, C. and Eastell, R. (2012) “Heart drugs that affect bone,” Trends Endocrinol Metab ,23(4):163-8.
Publisher – GoogleScholar
19. Wiens, M, Etminan, M. and Gill, S. S. (2006) “Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies,” J Intern Med , 260(4):350-362.
Publisher – GoogleScholar
20. Xia, J, Zhong, Y. and Huang, G. (2012) “The relationship between insulin resistance and osteoporosis in elderly male type 2 diabetes mellitus and diabetic nephropathy,” Ann Endocrinol (Paris) , 73(6):546-51.
Publisher – GoogleScholar
21. Yetkin, E, Turhan, H. and Senen, K. (2008) “Bone mineral density and frequency of coronary heart disease,” Am J Cardiol , 101(11):1680.
GoogleScholar






