Introduction
Infectious diseases still remain a crucial and challenging problem because of a combination of factors including rising infectious diseases and the increasing number of multi-drug resistant pathogens. Thus, there is still a need to discover new compounds with enhanced antimicrobial activities to combat drug resistance menace as corroborated by Jegede (2005). Paracetamol is a mild analgesic with weak anti-inflammatory activity, commonly used for the relief of aches and pains- Roberts et al (2001). However, overdose of Paracetamol may cause liver damage as validated by Larson et al (2005). Sulfamethoxazole is in the class of Sulfonamides, which are extensively used as antibacterial agent. This is due to the fact that they interfere with p-amino benzoic acid (PABA) in the biosynthesis of tetrahydrofolic acid, which is essential for the metabolic process of bacteria-Monti et al (2010). Sulfamethoxazole is a bacteriostatic antibiotic, used in combination therapy with Trimethoprim for the treatment of urinary tract infection. It is also used as an alternative to amoxicillin-based antibiotics in treating sinusitis and as prophylaxis of pneumonia in AIDS patient -Garg et al (1986). Furthermore, Streptomycin is a broad-spectrum, bactericidal antibiotic used in the treatment of tuberculosis in combination with other anti-TB drugs – Zhu et al (2001), and in combination with penicillin, it is used as a standard antibiotic cocktail to prevent bacterial infection in cell culture- Jan-Thorsten and Kee-Woei (2004). Its choice as a standard antibiotic in this study is influenced by its killing sensitive bacteria through stopping the production of essential proteins needed to survive- Zhu et al (2001). Mixed-ligand complexes containing nitrogen and oxygen atoms are of significant importance due to their antimicrobial and anticancer activities which are better than the metal-free ligands substantiated by Halli et al (2012) and Moustafa (2005). Similarly, many metal drug complexes have been found to have better antimicrobial and anticancer activities than classical drugs like cis-platin and Cloxacillin as validated by Harminder et al (2013); Lawal and Obaleye (2007); Nejo et al (2011); Osowole et al (2012); Osowole et al (2013a); Osowole et al (2013b); Bamigboye, et al (2012); Sadler and Zijien (1998).
Detailed literature search shows that mixed drug metal complexes of Sulfamethoxazole have been reported-Bamigboye et al (2012); Ma et al (2007); Monti et al (2010); Bellú et al (2005). However, no information is available on the mixed drug metal(II) complexes of Sulfamethoxazole and Paracetamol. Thus, we present the synthesis, characterization and antimicrobial activities of some novel metal(II) complexes of Sulfamethoxazole and Paracetamol, with the aims of investigating their magnetic properties for cooperative phenomenon such as antiferromagnetism, ferromagnetism and spin crossover. In addition, the potentials of these metal(II) complexes and their ligands as broad-spectrum antimicrobial agents in-vitro against pathogenic organisms will be investigated and compared with that of Streptomycin in order to discover suitable metal complexes for further research in metallo-antibiotic. This is a continuation of our group’s research on mixed drug metal complexes that could serve as lead compounds in drug research for pain and infection management -Osowole et al (2013a); Osowole et al (2013b); and Osowole et al (2012).
Experimental
Materials and Reagents
Reagent grade Cobalt(II) chloride hexahydrate, Copper(II) sulphate pentahydrate, Copper(II) nitrate trihydrate, Nickel(II) chloride hexahydrate, Manganese(II) nitrate hexahydrate, Zinc(II) sulphate heptahydrate, and Iron(II) sulphate heptahydrate were obtained from Aldrich and BDH chemicals. Paracetamol and Sulfamethoxazole were gifts from Bentos Pharmaceutical products limited, New Garage Ibadan and Mopson Pharmaceutical, Lagos, Nigeria and were used as received. Solvents were purified by distillation.
Preparation Of [Co (HL)(HL1)Cl2]. 2H2O
This complex was prepared by the addition of 0.47 g (1.974 x 10-3 moles) of CoCl2·6H2O to a stirring solution of 1.974 x 10-3 moles (0.30 g, paracetamol, HL) and 1.974 x 10-3 moles (0.50 g, Sulfamethoxazole, HL1) in 20 mL of methanol. The resulting homogeneous solution was then refluxed for 6 hours during which the product was formed. The pink precipitate obtained was filtered, washed with methanol and dried over silica gel. The same method was used for the preparation of the Mn(II), Fe(II), Ni(II), Cu(II) and Zn(II) complexes from their chloride, nitrate and sulphate salts respectively.
Physical Measurement
The electronic (solid reflectance) and infrared spectra (as KBr disc) of the complexes were recorded on a Perkin-Elmer λ25 and Perkin-Elmer FT-IR spectrum BX spectrometers in the range 4000-400 cm-1. Room temperature magnetic susceptibilities at 301K were measured on Sherwood Susceptibility Balance MSB Mark 1, melting points were determined with Mel-Temp electrothermal machine, molar conductance measurements of 1 x 10-3 M solutions in DMSO were obtained using electrochemical analyzer Consort C933 and percentage metal was determined by complexometric titration using EDTA.
Antimicrobial assay
The antimicrobial activities of the synthesized compounds as well as their metal free ligands were studied using the agar diffusion technique. The microbes used were identified clinical, food and environmental strains of Escherichia spp, Streptococcus spp, Proteus sp, Candida albicans, Salmonella sp, Bacillus spp, Staphylococcus sp and Pseudomonas sp. The surface of the agar in a Petri dish was uniformly inoculated with 0.2 mL of 18 hour old test bacterial culture. Using a sterile cork borer, 5 mm wells were bored into the agar. Then 0.06 mL of 10 mg/mL concentration of each metal complex in DMSO was introduced into the wells and the plates were allowed to stand on the bench for 30 minutes before incubation at 370C for 24 hours after which inhibitory zones (in mm) were taken as a measure of antimicrobial activity. The experiments were conducted in duplicates and Streptomycin was used as the reference drug.
Results and Discussion
The reaction of the Paracetamol (HL) and Sulfamethoxazole (HL1) with the metal(II) chlorides (Co and Ni), metal(II) nitrates (Mn and Cu) and metal(II) sulphates (Fe, Cu and Zn) gave coloured metal complexes, with moderate yields (30-40%) according to equations below.
NiCl2.6H2O + HL + HL1 → [Ni(HL)(HL1)Cl(H2O)]Cl.H2O + 4H2O ————————-(1)
2Cu(NO3)2.3H2O + 2HL + 2HL1→ [Cu(HL)(L1)NO3]2.H2O + 2HNO3 + 5H2O —————–(2)
MX2.aH2O + HL + HL1 → [M(HL)(HL1)X2].nH2O + cH2O —————–(3)
(when M = Mn, a = 6, n = 2, c = 4, X = NO3; M = Co, a = 6, n = 2 c= 4, X= Cl)
MSO4.aH2O + HL + HL1→ [M(HL)(HL1)(SO4)].nH2O + cH2O —————————-(4)
(when M = Fe, Zn, a = 7, n = 0, c=7; M = Cu, a = 5, n = 1, c =4)
The formation of the metal complexes was confirmed by percentage metal, distinct decomposition temperature, infrared and electronic spectroscopies. The ligands, Paracetamol (HL) and Sulfamethoxazole (HL1) melted at 170-1720C and 1690C respectively, whereas their metal complexes decomposed in the range 98-242 oC, confirming coordination. We have not been successful in our efforts to isolate single crystal of the metal complexes for X-ray diffraction measurements. The analytical data, colours, % metal, melting points, molar conductivity and room temperature magnetic moments for the complexes are presented in Table 1.
Percentage metal, Solubility and Conductance measurements
The experimental values of percentage metal in the complexes were in close agreement with the calculated values. This corroborated the proposed formula mass for the complexes.
The solubility of the metal complexes was tested in water, methanol, ethanol, nitromethane, DMSO and dichloromethane. However, the complexes were soluble only in DMSO. Consequently, the molar conductance of the metal (II) complexes was measured in DMSO and the values obtained were in the range 10.37– 23.1 Ω-1cm2mol-1 indicating their covalent nature, with the exception of the Ni(II) complex which had a value of 80.1 Ω-1cm2mol-1 indicative of a 1:1 electrolyte as validated by Geary (1971).
Electronic Spectra and Magnetic moments
The ultraviolet spectra of the HL (Paracetamol) and HL1 (Sulfamethoxazole) were characterized by a strong absorption maxima each at 32.68 and 32.79 kK respectively, assigned to π®π* transitions. These bands were shifted in the metal complexes to 30.0- 33.3 kK due to coordination (Table 2). The Mn(II) complex showed two absorption bands at 12.35 kK and 24.00 kK assigned to 6A1g ® 4Eg and 6A1g ® 4T1g transitions typical of octahedral geometry-Al-Saif and Refat (2012) . Literature showed that high spin octahedral Mn(II) complexes usually have moments close to spin only value of 5.90 B.M because orbital contribution is nil, a consequence of 6A1g ground term-Saha et al (2000). Thus, an observed moment of 5.94 B.M was corroborative of octahedral geometry -Saha et al (2000).
The Fe(II) complex had one absorption band at 23.98 kK typical of 6-coordinate, octahedral geometry and was assigned to 5T2g® 5Eg transition. Normally, high spin octahedral Fe(II) complexes have moments in the range 5.0-5.5 B.M while low spin octahedral Fe(II) complexes are diamagnetic. However, octahedral Fe(II) complexes are known to exhibit spin crossover, that is, equilibrium between the high spin 5T2g (t2g4 eg2) state and low spin 1A1(t2g6) state, with moments in the range 1.2-4.7 B.M- Matouzenko et al (2004). Consequently, the Fe(II) complex in this study had a moment of 1. 22 B.M corroborative of a high spin low spin octahedral equilibrium-Kitchen et al (2013) and Rudavskyi et al (2013).
The Co(II) complex exhibited three bands at 12.59, 21.60 and 23.75 kK which were consistent with octahedral geometry and were assigned to 4T1g(F) ® 4T2g, 4T1g(F) ® 4A2g and 4T1g(F) ® 4T1g(P) transitions. An observed moment of 4.57 B.M was supportive of octahedral geometry since moments in the range 4.6-5.2 B.M were reported for octahedral Co(II) complexes as validated by Housecroft and Sharpe (2005).
Furthermore, the Ni(II) complex showed two absorption bands at 14.93 and 23.98 kK typical of 6-coordinate octahedral geometry, assigned to 3A2g ® 3T1g(F) and 3A2g ® 3T1g(P) transitions. An observed moment of 3.13 B.M. was complimentary of octahedral geometry since moments in the range 2.8-3.3 B.M. were reported for octahedral Ni(II) complexes by Gupta and Sutar (2007).
The Zn(II) complex expectedly showed no d-d transition because it had d10 configuration. This complex was essentially diamagnetic and octahedral with a moment of 0.39 B.M. Similar result was obtained by Raman et al (2004).
The Cu(II) complexes, [Cu(HL)(L1)(NO3)]2.H2O and [Cu(HL)(HL1)(SO4)].H2O both had an absorption band each at 20.92 kK and 23.87 kK assigned to 2Eg → 2T2g transition of 6-octahedral, geometry as indicated by Agwara et al (2010). Mononuclear copper(II) complexes regardless of stereochemistry are expected to have effective magnetic moments in the range 1.9–2.2 B.M. usually higher than the spin only moment due to orbital contribution and spin-orbit coupling as validated by Gulcan et al (2012). Thus, [Cu(HL)(L1)(NO3)]2.H2O and [Cu(HL)(HL1)(SO4)].H2O had moments of 0.85 B.M. and 1.81 B.M. respectively. The latter compound’s moment of 1.81 B.M. was complimentary of octahedral geometry while the moment of 0.85 B.M. in the former compound was suggestive of anti-ferromagnetism operating through a Cu-Cu bond in a dimeric structure (Figure 1). However, we could not probe this further due to lack of facility for variable temperature magnetic moment measurement as validated by Singh et al (2012).
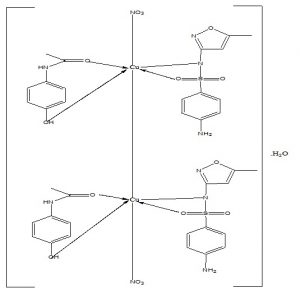
Figure 1: Propose structure for the dimeric copper(II) complex
Infrared Spectra
The strong and medium bands at 3467 cm-1 and 3376 cm-1 in Paracetamol and Sulfamethoxazole were assigned as υOH/(NH) and corroborated by Lawal and Obaleye (2007); Lutfar et al (2012) and Harminder et al (2013). The band at 3376 cm-1 in Sulfamethoxazole still remained in the metal(II) complexes but shifted to 3379-3393 cm-1, with the exception of [Cu(HL)(L1)NO3]2.H2O. This indicated coordination to the metal ion through nitrogen atom of the amine group – Bamigboye et al (2012). In the latter complex, the band at 3376 cm-1 in Sulfamethoxazole was absent due to de-protonation and coordination of the secondary nitrogen atom to the metal ion. Furthermore, the vOH band in Paracetamol, still showed in the metal complexes but shifted to 3406-3486 cm-1 due to coordination of the un-deprotonated phenol oxygen atom to the metal(II) ion as corroborated by Lawal and Obaleye (2007). The broad band at 3500 cm-1 in the Ni(II) complex was assigned to vOH water of coordination. Furthermore, the v(C=O) band of Paracetamol at 1622 cm-1 and 1595 cm-1 shifted to 1608-1625 cm-1 and 1545-1598 cm-1 in the metal complexes due to the coordination of the carbonyl oxygen atom. The v(S=O) (Sulphone) band at 1293 cm-1 (asymmetric) and 1149 cm-1 (symmetric) in Sulfamethoxazole shifted to 1279-1309 cm-1 and 1145-1164 cm-1 in the metal complexes due to the coordination of oxygen atom of the sulphone group. Additionally, the new bands in the range 522-586 cm-1 and 377-455 cm-1 and 356-371 cm-1, which were absent in the spectra of Paracetamol and Sulfamethoxazole, were assigned to υ(M-N), v(M-O) and v(M-Cl) respectively. Similar result was obtained by Al-Saif and Refat (2012) and Harminder et al (2013).
Antimicrobial activities
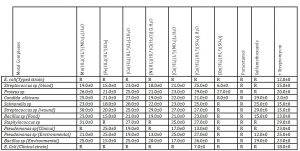
The antimicrobial activities of the ligands and their metal complexes are presented in Table 3. The complex, [Cu(HL)(HL1)(SO4)].H2O, was the only one with an activity of 7 mm against E. coli. The remaining metal complexes, Paracetamol and Sulfamethoxazole were not active against Escherichia spp. The ligand, Paracetamol, was inactive against all the tested bacteria while Sulfamethoxazole was active against six out of the tested microbes, that is, C. albicans, Salmonella sp, Streptococcus sp, Bacillus spp and Pseudomonas sp with inhibitory zones range of 12.0-29.0 mm. The [Cu(HL)(HL1)(SO4)].H2O had the best activity being active against all the microbes with the exception of E. coli(Typed strain) with inhibitory zones range of 7.0-29.0 mm. The next in activity were [Cu(HL)(L1)(NO3)]2.H2O and [Co(HL)(HL1)(Cl)2].2H2O, being active against all the microbes with the exceptions of Escherichia spp and inhibitory zones range of 17.0-25.0 mm and 19.0-28.0 mm respectively. These are followed in activity by [Mn(HL)(HL1)(NO3)2].H2O and [Fe(HL)(HL1)SO4] with activity against all the microbes with the exceptions of Escherichia spp and Pseudomonas sp(clinical) /Staphylococcus sp, and inhibitory zones range of 19.0-31.0 mm and 15.0-25.0 mm respectively. The complex, [Ni(HL)(HL1)Cl(H2O)]Cl.H2O was next in activity, being active against all the microbes with the exceptions of Escherichia spp, Pseudomonas sp and Staphylococcus sp with inhibitory zones range of 13.0-29.0 mm. The Zn(II) complex had the lowest activity being active against three organisms namely Streptococcus sp, Proteus sp and C. albicans with inhibitory zones range of 6.0-27.0 mm. Its lowest activity was attributed to a probable lipophobic nature which made permeation though lipid bacteria membrane impossible as indicated by Weder et al (2002). Generally, the metal(II) complexes were mostly more effective than the metal free drugs, Paracetamol and Sulfamethoxazole, due to chelation which increases lipophilic character, favouring its permeation through lipid layers of the bacterial membrane as documented by Agwara et al (2010). The non-activity of Paracetamol against tested microbes indicated that it was not toxic at 10mg/mL and also confirmed its uses as pain killer as indicated by Harminder et al (2013). It was interesting to note that the mixed drug metal complexes were mostly more active than Streptomycin, and Streptomycin was expectedly active against all the tested microbes with inhibitory zones range of 2.0-29.0 mm.
Table 1: Analytical data of complexes
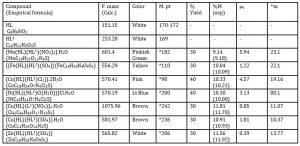
Table 2 Relevant infrared and electronic spectra data of the complexes
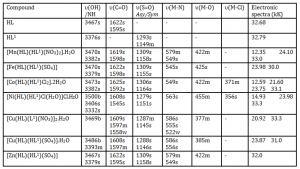
Table 3 : Antibacterial activities of the ligands and their complexes
Conclusion
Mixed drug Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of Paracetamol and Sulfamethoxazole, were synthesized and characterized by infrared and electronic spectroscopies, room temperature magnetic moments, melting points and conductance measurements. Electronic spectra and room temperature magnetic moment data corroborated octahedral geometry for the metal complexes, with Cu(II) nitrate metal complex being dimeric. The conductance measurements in DMSO showed that only the Ni(II) complex was a 1:1 electrolyte. The in-vitro antimicrobial studies of the complexes against Escherichia spp, Proteus sp, C. albicans, Salmonella sp, Streptococcus spp, Bacillus spp, Staphylococcus sp, Pseudomonas sp showed that [Co(HL)(HL1)Cl2].2H2O, [Cu(HL)(L1)(NO3)]2.H2O and [Cu(HL)(HL1)SO4].H2O had broad spectrum antimicrobial activities against all the microbes with the exceptions of Escherichia spp.
(adsbygoogle = window.adsbygoogle || []).push({});
References
- Agwara, M. O., Ndifon, P. I., Ndosiri, N. B., Paboudam, A. G., Yufanyi, D. M. and Mohamadou,A. (2010) “Synthesis, characterization and antimicrobial activities of Co(II), Cu(II) and Zn(II) mixed – Ligand complexes containing 1,10-phenanthroline and 2, 2’- bipyridine,”Bulletin of the Chemical Society of Ethiopia, 24 (3) 383-389.
- Al-Saif, F. A. and Refat, M. S. (2012) “Ten metal complexes of vitamin B3/niacin: Spectroscopic, thermal, antibacterial, antifungal, cytotoxicity and antitumor studies of Mn(II), Fe(III),Co(II), Ni(II), Cu(II), Zn(II), Pd(II), Cd(II), Pt(IV) and Au(III) complexes,” Journal of Molecular Structure, 1021 40-52.
- Bamigboye, M. O., Obaleye, J. A. and Abdulmolib, S. (2012) “Synthesis, characterization and antimicrobial activity of some mixed sulfamethoxazole-cloxacillin metal drug complexes,” International Journal Chemistry, 22 (2) 105-108.
- Bellú, S., Hure, E. M., Trapé, M., Trossero, C., Molina, G., Drogo, C., Williams, P. A. M., Atria, A. M., Muñoz Acevedo, J. C., Zacchino, S., Sortino, M., Campagnoli, D. and Rizzotto, M. (2005) “Synthesis, Structure and Antifungal Properties of Co (II)-sulfathiazolate complexes,” Polyhedron 24 501-509.
- Garg, S. K., Ghosh, S. S. and Mathur, V. S. (1986) “Comparative pharmacokinetic study of four different sulfonamides in combination with trimethoprim in human volunteers,” Toxicology 24 (1) 23-5. PMID 3485584.
- Geary, W. J. (1971) “The use of conductivity measurements in organic solvents for the characterization of coordination compounds,” Coordination Chemistry Revised, 7 81-122.
- Gulcan, M., Sonmez, M. and Berber, I. (2012) “Synthesis, characterization and antimicrobial activity of a new pyrimidine Schiff base and its Cu(II), Ni(II), Co(II), Pt(II) and Pd(II) complexes,” Turkish Journal of Chemistry, 36 189-200.
- Gupta, K. C. and Sutar, A. K. (2007) “Polymer supported Schiff base complexes of iron(III),cobalt(II) and nickel(II) ions and their catalytic activity in oxidation of phenol and cyclohexane,” Journal of Macromolecular Science: A Pure Applied Chemistry, 44 (11) 1171–1185.
- Halli, M. B., Patil, V. B., Sumathi, R. B. and Mallikarjun, K. (2012) “Synthesis, characterization and biological activity of mixed ligand metal(II) complexes derived from benzofuran-2 carbohydrazide Schiff base and malonyldihydrazide,” Der Pharma Chemica, 4 (6) 2360-2367.
- Harminder, K., Kanav, D., Jaspreet, K., Bharti, M. and Ashish, C. (2013) “Synthesis and evaluation of diorganotin(IV) and triorganotin(IV) derivatives of Aspirin, Paracetamol and Metroni dazole as antimicrobial agents,” American Journal of drugs and development, 3 (1) 13-22.
- Housecroft, C. E. and Sharpe, A. G. (2005) Inorganic Chemistry, Ashford Colour Press Ltd., Gosport.
- Jan-Thorsten, S. and Kee-Woei, N. G. (2004) “A manual for primary human cell culture,” World Scientific, 89.
- Jegede, C. A. (2005) “Synthesis, Characterization and Biological studies of some metal fluoroquinolone complexes,” PhD Thesis, Unilorin Nigeria.
- Kitchen, J. A., Olguín, J., Kulmaczewski, R., White, N. G., Milway, V. A., Jameson, G. N. L., Tallon, J. L. and Brooker, S. (2013) “Effect of N4 substituent choice on spin crossover in dinuclear Iron (II) complexes of bis-terdentate-1,2,4-triazole-based,” Inorganic Chemistry, DOI:10.1021/ ic4014416.
- Larson, A. M., Polson, J. and Fontana, R. J. et al. (2005) “Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study,” Hepatology, 42 (6) 1364–1372.
- Lawal, A. and Obaleye, J. A. (2007) “Synthesis, characterization and antibacterial activity of aspirin and paracetamol-metal complexes,” Biokemistri, 19 (1) 9-15.
- Lutfar, K., Rahman, K. L., Mamun, M. A. and Ehsan, M. Q. (2012) “Preparation of metal Niacin complexes and characterization using spectroscopic and electrochemical techniques,” Russian Journal of Inorganic Chemistry, 56 (9) 1436-1442.
- Ma, M., Cheng, Y., Xu, Z., Xu, P., Qu, H., Fang, Y., Xu, T. and Wen, L. (2007) “Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug,” European journal of medicinal chemistry, 42 (1) 93–8. doi:1016/j.ejmech.2006.07.015. PMID17095123.
- Matouzenko, G.S., Bousseksou, A., Borshch, S.A., Perrin, M., Zein, S., Salmon, L., Molnar, G. and Lecocq, S. (2004) “Cooperative spin crossover and order-disorder phenomena in a mononuclear compound [Fe(DAPP)(abpt)](ClO(4))(2) [DAPP = [bis(3-aminopropyl)(2-pyridylmethyl)amine], abpt = 4-amino-3,5-bis(pyridin-2-yl)-1,2,4-triazole],” Inorg Chem., 12 43(1) 227-236.
- Monti, L., Ana Pontoriero N. M., Giulidori, C., Hure, E. and Patricia, A. M. W., Rodríguez, M. V., Feresin, G., Campagnoli, D. and Rizzotto, M. (2010) “Synthesis, characterization and antimicrobial properties of a Co(II)-phthalylsulfathiazolate complex,” Biometals, 23 1015-1028.
- Moustafa, M. E. (2005) “Synthesis and structural and biological activity studies on some lanthanide chelates with O- and N-containing ligands,” Spectroscopy Lett., 38 (1) 23–34.
- Nejo, A. A., Kolawole, G. A., Nejo, A. O. and Muller, C. J. (2011) “Synthesis, characterization and insulin-mimetic studies of complexes of Oxovanadium(IV) with Schiff bases,” International Journal of drug formulation and research, 2 (5) 220-241.
- Osowole, A. A., Ekennia, A. C. and Achugbu, B. O. (2013a) “Synthesis, spectroscopic characterization and antibacterial Properties of some Metal (II) Complexes of 2-(6-methoxybenzothiazol-2-ylimino)methyl)-4-nitrophenol,” Research & Reviews: Journal of Pharmaceutical Analysis, 2 (2) 1-5.
- Osowole, A. A., Ekennia, A. C., Achugbu, B. O. and Etuk, G. H. (2013b) “Synthesis, spectroscopic characterization and structure related antibacterial activities of some metal(II) complexes of substituted triflurobutenol,” Elixir Applied Chemistry, 59 15848-15852.
- Osowole, A. A., Oni, A. A., Onyegbula, K. and Hassan, A. T. (2012) “Synthesis, spectral, magnetic and in-vitro anticancer properties of some Metal(II) complexes of 3-[2,4-dihydro-1H-inden-4-ylimino)methyl]napthalen-2-ol,” International Research Journal of Pure and Applied Chemistry, 2 (3) 211-220.
- Raman, N., Ravichandran, S. and Thangaraja, C. (2004) “Cu(II), Co(II), Ni(II) and Zn(II) complexes of Schiff base derived from benzil-2,4-dinitrophenyl hydrazone with aniline,” Journal of Chemistry Society (Indian Academic Society), 116–215.
- Roberts, L., Jackson, M., Jason, D. (2001) “Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout,” In Gilman, Alfred; Goodman, Louis Sanford; Hardman, Joel G.; Limbird, Lee E.. Goodman & Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill, 687–732.
- Rudavskyi, A., Havenith, R. W. A., Broer, R., Graaf, C. and Sousa, S. (2013) “Explanation of the site-specific spin crossover in [Fe(mtz)6(BF4)2],” Dalton Transaction, DOI:10.1039/ c3dt52027g
- Sadler, P. J. and Zijien (1998) “Metal complexes in medicine, Designed and Mechanism of Action,” Journal of Pure and Applied Chemistry, 4 70.
- Saha, A., Majumdar, P. and Goswami, S. (2000) “Low-spin manganese(II) and cobalt(III) complexes of N-aryl-2-pyridyl azophenylamines: new tridentate N,N,N-donors derived from cobalt mediated aromatic ring amination of 2-(phenylazo)pyridine. Crystal structure of a manganese(II) complex,” Journal of Chemistry Society Dalton Transaction, 11 1703-1708.
- Singh, K., Kumar, Y., Puri, P. and Singh, G. (2012) “Spectroscopic, Thermal, and Antimicrobial Studies of Co(II), Ni(II), Cu(II), and Zn(II) Complexes Derived from Bidentate Ligands Containing N and S Donor Atoms,” Bioinorganic Chemistry and Applications, 2012 1-9.
- Weder, J. J. E., Dillon, C. T., Hambley, T. W., Kennedy, B. J., Lay, P. A., Biffin, J. R., Regtop, H. L. and Davies, N. M. (2002) “Copper complexes of non-esteroidal anti-inflammatory drugs: an opportunity yet to be realized,” Coordination Chemistry Reviews, 232 95-126.
- Zhu, M., Burman, W. J., Jaresko, G. S., Berning, S. E., Jelliffe, R.W. and Peloquin, C. A. (2001)“Population pharmacokinetics of intravenous and intramuscular streptomycin in patients with tuberculosis,” Pharmacotherapy, 21 (9) .






