Introduction
The coordination chemistry of mixed ligand complexes provide new compounds that can act as catalysts in reactions of industrial importance such as hydrogenation, hydroformation, and oxidative hydrolysis of olefins and carboxylation of methanol as shown by studies carried out by Hronec et al., in 1987. Syed and Leal in 2008 demonstrated that Meta-Toluic acid is a precursor to DEET (N, N-diethyl-m-toluamide), a well-known insect repellent while Niacin is pharmacologically and physiologically active .e.g. it is a major component of important coenzymes used in DNA repair and cell signaling as confirmed by various research studies- Bruckert et al., (2010), Lucasova et al., (2011), Taylor et al., (2006), Villins et al., (2012), and Wu et al., (2010). Furthermore, Niacin can be described as a biological chelating ligand due to the existence of nitrogen and oxygen atoms on its structure that can act as coordinating sites for metal ions coordination. Detailed literature search revealed that few research works such as Dillip et al., (2011), Sayyed and Abdulrahim (2012), and Vaskova et al., (2009) has been done on the metal(II) complexes of niacin, however no information is available on metal(II) mixed ligand complexes of niacin and m-toluic acid. Thus, our aim is to synthesize, characterize and investigate the magnetic properties of these novel metal(II) complexes for cooperative phenomenon like ferromagnetism, ferrimagnetism and antiferromagnetism. Secondly, the potentials of these metal complexes as broad-spectrum antibacterial agents in-vitro will also be verified, as a continuation of our research in the field of bioinorganic chemistry as corroborated by the following researches: (Osowole et al., 2014, 2013a, 2013b, 2012a, 2012b).
Experimental
Materials and reagents
Reagent grade m-Toluic acid, Niacin, Copper(II) chloride dihydrate, Nickel(II) chloride hexahydrate, Cobalt(II)chloride hexahydrate, Manganese(II) chloride tetrahydrate, Iron(II) sulphate heptahydrate, Zinc(II) nitrate hexahydrate and Zinc(II) acetate tetrahydrate were obtained from Aldrich chemicals, and solvents were purified by distillation.
Preparation Of [Mn(HL)(HL1)Cl2].H2O
0.5 g (4.06 mmole) of Niacin (HL) and 0.55 g (4.06 mmole) m-Toluic (HL1) are dissolved in 30 mL of methanol. To the resulting homogenous solution, 0.8 g (4.06 mmole) of the Mn(II) Chloride tetrahydrate was added while stirring and heating at 60oC. The resulting homogenous solution was then refluxed for 3 h, during which the product formed. This was filtered, washed with methanol and dried over silica gel. The same procedure was used for the preparation of Co(II), Ni(II), Cu(II), Fe(II), and Zn(II) complexes from their chloride, nitrate and sulphate salts respectively.
Physical Measurement
The molar conductance measurements of 1 x 10-3 M solutions in DMSO and the electronic spectra of the complexes in DMSO were recorded using electrochemical analyzer Consort C933 and a Perkin-Elmer λ25 spectrophotometer respectively. The infrared spectra were recorded on a Perkin-Elmer FT-IR spectrum BX spectrometer in the range 4000-400 cm-1 as KBr disc while melting points were determined with Mel-Temp electrothermal machine, and the room temperature magnetic susceptibilities at 303K were measured with Sherwood Susceptibility Balance MSB Mark 1.
Antibacterial Assay
The antibacterial activity of the metal complexes and their ligands against laboratory strains of Bacillus cereus, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella oxytoca and Staphylococcus aureus were determined using the agar diffusion technique. The surface of Muller Hinton’s, agar in a petri dish was uniformly inoculated with 0.2 mL of 18 hour old test bacterial culture and with a 9 mm sterile cork borer, wells were bored into the agar, followed by the addition of 0.06 mL of 10 mg/mL of each metal complex in DMSO. The plates were incubated at 370C for 24 hours, after being allowed to stand on the bench for 30 minutes and inhibitory zones (in mm) were taken as a measure of antibacterial activity. The experiments were conducted in duplicates and Augmentine was used as the reference drug.
Results and Discussion
The reaction of the Niacin (HL), m-Toluic acid (HL1) with the metal(II) chlorides (Mn, Ni, Cu and Co) and Metal(II) nitrate and acetate of Zn and sulphate of Fe give coloured complexes in good yields according to equations 1- 3.
MCl2.aH2O + HL + HL1→ [M(HL)(HL1)Cl2].H2O + bH2O —————– (1)
(when M = Mn, a = 4, b = 3; Co, Ni; a = 6, b = 5)
Zn(X)2.aH2O +HL + HL1 → [Zn(HL)(HL1)X2].nH2O + bH2O ———————- (2)
(when X = NO3–. a =6, b = 5, n = 1; X= CH3COO–, a = 4, b = 2, n=2)
FeSO4.7H2O +HL + HL1 → [Fe(HL)(HL1)SO4] + 7H2O ——————— (3)
The ligands, Niacin (HL) and m-Toluic acid (HL1), melt at 237 0C, and 108-110 0C respectively, whereas their metal complexes all melt or decompose in the range 100- 260 0C, confirming coordination (Table 1). The formation of the metal complexes is further confirmed by percentage metal, infrared and electronic spectroscopies. In the absence of suitable crystal for single x-ray diffraction measurement, percentage metal, magnetic and spectroscopic data were used to propose possible structures (Figure 1).
Solubility And Conductance Measurements
The complexes are mostly insoluble in water, methanol, ethanol, nitromethane, and chloroform but are soluble in DMSO. Consequently, their molar conductances are measured in DMSO with values obtained in the range 3.05–41.90 Ω-1cm2mol-1 indicating their covalent nature as validated by Geary (1971).
Electronic Spectra and Magnetic moments
The ultraviolet spectra of the HL (Niacin) and HL1 (m-Toluic acid ) are characterized by strong absorption maxima in the range 25.58 kK and 33.22- 35.34 kK, with molar extinction coefficient in the range 104–105 cm2 mol-1, respectively assigned to n®π* and π®π* transitions. In the metal complexes, these bands shifted to the range 35.71-38.78 kK and 40.0-43.47 kK respectively due to coordination (Table 2).
The Mn(II) complex shows two absorption bands at 16.67 kK and 23.53 kK assigned to 6A1g →4T2g (G), and 6A1g →4Eg(G). Octahedral Mn(II) complexes are expected to have moments close to the spin-only value of 5.90 B.M since the ground term is 6A1g and as such there is no orbital contribution. Consequently, a moment of 6.16 B.M. observed for this complex indicates that it is high spin and complementary of octahedral geometry, similar result was obtained by Durot et al., (2003).
The Fe(II) complex has a single absorption band at 23.81 kK typical of 6-coordinate, high spin octahedral geometry and is assigned to 5T2g → 5Eg transition. A moment of 5.0-5.5 B.M is usually observed for high spin complex Fe(II) complexes. In this study, a moment of 5.87 B.M is observed for this complex, which is complimentary of high spin octahedral geometry, substantiated by Adetoye et al., (2009).
The Co(II) complex exhibits three bands at 14.71, 17.86, and 23.55 kK, assigned to 4T1g(F)→4T2g(F), (ν1), 4T1g(F)→ 4A2g(F), (ν2), and 4T1g(F) → 4T1g(P), (ν3), transitions of an octahedral geometry. The observed moment of 4.99 B.M is complimentary of octahedral geometry since moments in the range 4.7–5.2 B.M is usually observed for octahedral Co(II) compounds, this result is supported by the research of Mashalay et al., (2007).
The Ni(II) complex shows absorption bands at 18.69 and 23.53 kK assigned to 3A2g(F) →3T1g(F), (ν2), and 3A2g(F) →3T1g(P), (ν3),transitions, in an octahedral environment. Generally, square planar Ni(II) complexes are diamagnetic, while distorted octahedral Ni(II) complexes are paramagnetic with moments in the range 3.70–4.20 B.M., this is verified by Abd El-Wahab, (2008) with complexes of similar magnetic moments. The complex in this study gives a moment of 3.77 B.M. and hence is octahedral (Figure 1a).
The observance of two unsymmetrical bands at 11.75 and 23.42 kK in the Cu(II) complex is typical of a 6-coordinate, tetragonal (octahedral) geometry and are assigned to 2B1g → 2B2g and 2B1g → 2Eg transitions confirmed by Gulcan et al., (2012) since regular octahedral Cu(II) complexes have single band between 10-0-20.0 kK. Mononuclear copper(II) complexes, regardless of geometry, usually have moments in the range 1.9–2.2 B.M, higher than spin-only moment of 1. 73 B.M due to orbital contribution and spin-orbit coupling. The Cu(II) complex in this study, has a moment of 1.69 B. M, which is lower than expected due to antiferromagnetism as validated by Osanai et al., (2006), operating through metal- metal bond in a dimeric structure (Figure 1b) . However, we are unable to confirm this due to the absence of variable temperature magnetic moment meter and viable crystals for single crystal X-ray structural investigation.
The Zn(II) complexes, [Zn(HL)(HL1)(CH3CO2)].H2O and [Zn(HL)(HL1)(NO3)].H2O show only the CT transitions from M → L, as no d-d transition is expected around 23.40 kK. The former is expectedly diamagnetic whereas the latter has a moment of 0.70 B.M due to polarisation paramagnetism, and they assume a 6-coordinate octahedral geometry -Onal et al., (2011) and Raman et al (2001).
Octahedral and tetrahedral complexes may be easily distinguished based on their molar extinction coefficient (Ɛ), that is, the former usually have Ɛ between 1–50 cm2 mol-1 while the latter exhibits a higher molar extinction coefficient in the range 102–103 cm2 mol-1 because they are asymmetric. Thus, in this study all the metal complexes have Ɛ of 10-60 cm2 mol-1, indicative of octahedral geometry and were corroborated by Nejo et al., (2011).
Infrared Spectra
The strong and broad bands at 3374 cm-1 and 3008 cm-1 in Niacin and m-Toluic acid are assigned as υOH band (Table 2). Its broad band is due to hydrogen bonding which is very strong in carboxylic acid; this was corroborated by Obaleye et al., (2011). These bands remain in the metal complexes but are shifted to 3512-3301 cm-1, indicating coordination through un-deprotonated OH. The vC=O band in Niacin at 1627 cm-1 shifted only marginally in the complexes to 1626-1624 cm-1 indicating non-coordinating of the carbonyl oxygen atom. On the contrary, the vC=O band in m-Toluic acid at 1687 and 1590 cm-1 shift to 1706-1605 cm-1 in the metal complexes, confirming the coordination of the carbonyl oxygen atom to the metal ions -Nejo et al., (2011). The broad band at 3500 cm-1 in the metal complexes are assigned to v(OH) of coordinated water, as confirmed by Chadar and Khan (2006). Furthermore, the new bands in the range 586 – 511 cm-1 and 491-400 cm-1 and 396-370cm-1, which are absent in Niacin and m-Toluic acid, are assigned to υ(M-N), v(M-O) and v(M-Cl) respectively, similar results were obtained by Al-Saif and Refat (2012).
Antibacterial Activities
Niacin and the Fe(II) complex have no activity against the bacteria used (Table 3). The Cu(II) complex has the least activity being active against four organisms i.e E. coli, B. cereus, P. mirabilis and S. aureus with inhibitory zones range of 15.0-23.0 mm. This is followed in activity by the Mn(II) and Zn(II) complexes, which have activities against five organisms; E. coli, B. cereus, P. mirabilis, S. aureus and K. oxytoca inhibitory zones range of 13.0-25.0 and 21.0-35.0 mm respectively. The Co(II) and Ni(II) complexes have the best antibacterial activities being active against all the bacteria used with inhibitory zones range 21.0- 49.0 mm and 21.0-37.0 mm respectively. Thus, proving their potentials as broad-spectrum antibacterial agent. Generally, the metal(II) complexes are mostly more effective than the metal-free ligands, Niacin and m-Toluic acid, due to chelation which increases lipophilic character, favouring its permeation through lipid layers of the bacterial membrane according to Obaleye et al., (2011). Conversely, the inactivity of Niacin and the Fe(II) complex may be attributed to their lipophobic character as documented by Abd El-Wahab (2008). Expectedly, Streptomycin is more active than the metal complexes against P. aeruginosa, B. cereus and S. aureus with inhibitory zones range of 45.0-67.0 mm. However, with E. coli, K. oxytoca, and P. mirabilis, the Co(II) complex has a higher activity than streptomycin (21.0-33.0 mm) with inhibitory zones range of 35.0-49.0 mm. This we cannot explain.
Conclusion
Mixed ligand metal(II) complexes of Niacin (HL) and m-Toluic acid (HL1), have been synthesized and characterized by percentage metal, infrared and electronic spectroscopies, room temperature magnetic moments, melting points and conductance measurements. The complexes are all covalent, with a 6-coordinate octahedral geometry. The room temperature magnetic moment confirms that the metal complexes are magnetically dilute with the exception of the Cu(II) complex which is antiferromagnetic with a room temperature magnetic moment of 1.69 B.M. The Co(II) and the Ni(II) complexes have broad-spectrum antibacterial activities against B. cereus, E. coli, P. mirabilis, P. aeruginosa, K. oxytoca and S. aureus with inhibitory zones range of 21.0-49.0 mm and 21.0-37.0 mm respectively, proving their potentials as broad spectrum antibacterial agent.
Table 1: Analytical data of the ligands and complexes
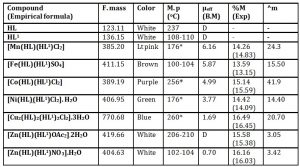
HL = Niacin, HL1 = m-Toluic acid, D = diamagnetic, *= decomposition temperature, Lt = light, Molar conductance (^m) = ohm−1 cm2 mol−1, Exp = experimental, F. mass = Formular mass,
µeff = effective magnetic moments, M. p = melting point.
Table 2: Relevant infrared and electronic spectra data of the Complexes
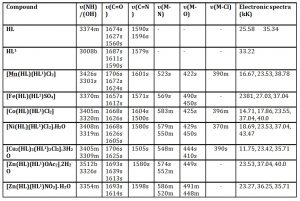
HL = Niacin, HL1 = m-Toluic acid, b = broad, s = strong, m= medium, w= weak; 1kK = 1000cm-1
Table 3: Antibacterial activity data of the ligands and complexes
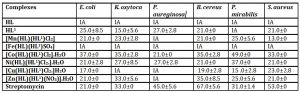
Results are expressed as means (± error) of duplicate experiments, HL = Niacin, HL1= m-Toluic acid, IA= Inactive
(adsbygoogle = window.adsbygoogle || []).push({});
References
- Abd El-Wahab, Z. H. (2008) “Complexation of 4-amino-1, 3-dimethyl-2, 6 pyrimidine dione derivatives with cobalt(II) and nickel(II) ions: synthesis, spectral, thermal and antimicrobial studies,” Coord. Chem., 61 (11) 1696-1709.
- Adetoye, A. A., Egharevba, G. O., Obafemi, C. A. and Kelly, D. R. (2009) “Synthesis and physicochemical properties of Co(II), Cu(II), Fe(III), Mn(II), and Ni(II) complexes of the isatin derivative of sulphanilamide,” Toxicological & Environmental Chemistry, 91 (5) 837–846.
- Al-Saif, F. A and Refat, M. S. (2012) “Ten metal complexes of vitamin B3/niacin: Spectroscopic,thermal, antibacterial, antifungal, cytotoxicity and antitumor studies of Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Pd(II), Cd(II), Pt(IV) and Au(III) complexes,”Journal of Molecular Structure, 1021 40-52.
- Bruckert, E., Labreuche, J. and Amarenco, P. (2010) “Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis,” Atherosclerosis, 210 (2) 353-361.
- Chadar, S. N and Khan, F (2006) “Electrode Kinetics and Ternary Complexes of [MnII-antibiotics-vitamine-B2] vis-à-vis Kinetics of Electrode Reaction,” J Indian Chem Soc., 83 1242-1247.
- Dilip, C. S., Venkatachalam, K. J., Raj, A. P. and Ramachandramoorth,T (2011) “Microwave assisted synthesis and structural characterisation of nicotinic acid and nitrito–κo mixed ligand complexes,” J. of Life and Pharma Sci., 1 1-9.
- Durot, S., Policar, C., Pelosi, G., Bisceglie, F., Mallah, T. and Mahyt, J. P. (2003) “Structural and magnetic properties of carboxylatobridged manganese(II) complexes involving tetradentate ligands: Discrete complex and 1D polymers. Dependence of J on the nature of the carboxylato bridge,” Inorganic Chemistry, 42 (24) 8072–8080.
- Geary, W. J.(1971) “The use of conductivity measurements in organic solvents for the characterization of coordination compounds,” Chem. Rev., 7 81–122
- Gulcan, M., Sonmez, M., and Berber, I. (2012) “Synthesis, characterization and antimicrobial activity of a new pyrimidine Schiff base and its Cu(II), Ni(II), Co(II), Pt(II) and Pd(II) complexes,” Turkey Journal of Chemistry, 36 189-200.
- Hronec, M., Masarovič, F., Cvengrošová, Z. and Ilavský , J. (1987) “Oxidation of p–xylene to terephthalic acid in benzoic acid and methyl ester of p–toluic acid,” Czech. Chem. Commun., 52 2241-2247.
- Lukasova, M., Hanson, J., Tunaru, S. and Offermanns, S. (2011) “Nicotinic acid (niacin): New lipid-independent mechanisms of action and therapeutic potentials,” Trends in Pharmacological Sciences, 32 (12) 700-707.
- Mashalay, M. M., Seleem, H. S., El-Behairy, M. A. and Habib, H. A. (2007) “Thermal, spectral, magnetic and biological studies of thiosemicarbazone complexes with metal ions: Cu(II), Co(II), Ni(II), Fe(III), Cd(II), Zn(II), Mn(II) and UO2(VI),” Polish Journal of Chemistry, 78 (11–12) 2055–2074.
- Nejo, A. A., Kolawole, G. A., Nejo, A. O. and Muller, C. J. (2011) “Synthesis, characterization and insulin-mimetic studies of complexes of Oxovanadium(IV) with Schiff bases,” International Journal of Drug Formulation and Research, 2 (5) 220-241.
- Obaleye, J. A., Adediji, J. F. and Adebayo, M. A. (2011) “Synthesis and biological activities on metal complexes of 2, 5-Diamino-1, 3, 4-thiadiazole derived from semicarbazide hydro chloride,” Molecules, 16 5861-5874.
- Onal, Z., Zengin, H. and Sonmez, M. (2011) “Synthesis, characterization and photoluminescence properties of Cu(II), Co(II), Ni(II) and Zn(II) complexes of N-aminopyrimidine-2-thione,” Turkey Journal of Chemistry, 35 905-914.
- Osanai, K., Okazawa, A., Nogami, T., and Ishida, T.(2006) “Strong ferromagnetic exchange couplings in Cu(II) and Ni(II) complexes with a paramagnetic tridentate chelate ligand, 2,2_-bipyridin-6-yl tert-butyl nitroxide,” Am. Chem. Soc., 128 14008–14009.
- Osowole, A. A., Ekenia, A. C. and Achugbu, B. O. (2013a) “Synthesis, spectroscopic characterization and antibacterial properties of some metal(II) Complexes of 2-(6-methoxybenzothiazol-2-ylimino)methyl)-4-nitrophenol,” Research & Reviews: Journal of Pharmaceutical Analysis, 2 (2) 1-5.
- Osowole, A. A., Ekennia, A. C., Achugbu, B. O. and Etuk, G. H (2013b) “Synthesis, spectroscopic characterization and structure related antibacterial activities of some metal(II) complexes of substituted triflurobutenol,” Elixir Appl Chem., 59 15848-15852
- Osowole, A. A., Ekennia, A. C. and Osukwe, A. E. (2014) “Synthesis, spectroscopic and antibacterial properties of some metal (II) mixed ligand complexes of Riboflavin and 2,2’-Bipyridine,” Research and Reviews: Journal of Chemistry, 3 (1) 32-37.
- Osowole, A. A., Oni, A. A., Onyegbula, K. and Hassan, A. T. (2012a) “Synthesis, spectral, magnetic and in-vitro anticancer properties of some Metal(II) complexes of 3-[2,4-dihydro-1H-inden-4-ylimino)methyl]napthalen-2-ol,” International Research Journal of Pure and Applied Chemistry, 2 (3) 211-220.
- Osowole, A. A. and Ott, I. (2012b) “Synthesis, characterization, in-vitro anticancer and antimicrobial properties of some metal(II) complexes of 4-[(2,3-dihydro-1H- inden-4-ylimino) methyl] benzene-2, 4-diol,” International Research Journal of Pure and Applied Chemistry, 2 (2) 156-169.
- Raman, N., Kulandaisamy, A. and Jeyasubramanian, K. (2001) “Synthesis, spectroscopic characterization, redox and biological screening of some Schiff base transition metal(II) complexes derived from salicylidene-4-aminoantipyrine and 2-aminophenol/2-amino thio phenol,” React. Inorg. Met.-Org.Chem., 31 (7) 1249–1270.
- Sayyed, H and Abdulrahim, M. F. (2012) “Studies of binary complexes of bivalent metal ions with Nicotinic acid by Potentiometry,” Adv. Sci. Res., 3 (4) 68-69.
- Syed, Z and Leal, W. S. (2008) “Mosquitoes smell and avoid the insect repellent DEET,” Natl. Acad. Sci., (USA) 105 (36) 13598–13603.
- Taylor, A. J., Lee, H. J. and Sullenberger, L. E (2006) “The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3,” Current Medical Research and Opinion, 22 (11) 2243-2250.
- Vasková, Z., Stachová, P., Krupkováa, L., Hudecováa, D and Valigura, D. (2009) “Bis(nitrobenzoato) copper(II) Complexes with Nicotinamide, Preparation, Structure and Properties,” Acta Chimica Slovaca, 2 (1) 77 – 87.
- Villines, T. C., Kim, A. S., Gore, R. S. and Taylor A. J. (2012). “Niacin: The evidence, clinical use and future directions,” Current Atherosclerosis Reports, 14 (1) 49-59.
- Wu, B. J., Yan, L., Charlton, F., Witting, P., Barter, P. J. and Rye, K. A.(2010) “Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids,” Atherosclerosis, Thrombosis, and Vascular Biology, 30 (5) 968-975.





