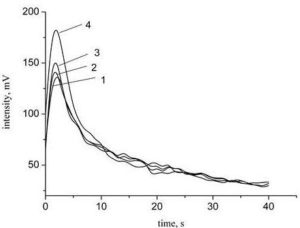
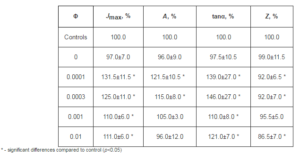
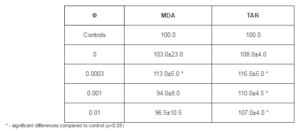
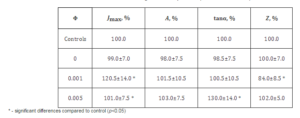
In addition, it can be seen in Tables 1 and 3 that at the same concentration of fullerene (Φ=0.001) the value of tanα is higher for the film formed of o-xylene than for the film formed of toluene. This can be explained by the fact that the solubility of fullerenes in o-xylene is higher than in toluene, what correlates with the findings of Zhou et al (1997). It appears that in toluene solution the fullerene molecules are in the form of clusters, which does not ensure uniform distribution of the nanoparticles in the composite film during its formation of solution.
Note also, we had preliminary experiments with films containing fullerene that were fabricated by the casting of aliphatic compound — chloroform. The results of chemiluminescent analysis and spectrophotometry for serum samples after exposure of both original polystyrene film and composite films regardless of the fullerenes content were approximate to controls. This again emphasizes the significance of the medium in which the films were fabricated.
In conclusion, our investigation proved that polystyrene/fullerene nanocomposites have the ability to activate lipid peroxidation in blood serum. Moreover, the possibility of such activation depends on the composite forming conditions.
Acknowledgments
The study was supported by the Russian Foundation for Basic Research (project no. 12-03-97528-a).
References
Alekseeva, O. V., Bagrovskaya, N. A., Kuz’min, S. M, Noskov, A. V., Melikhov, I. V. & Rudin, V. N. (2009). “The Influence of Fullerene Additives on the Structure of Polystyrene Films,” Russian Journal of Physical Chemistry A, 83 (7) 1170-1175.
Publisher – Google Scholar
Andreev, I., Petrukhina, A., Garmanova, A., Babakhin, A., Andreev, S., Romanova, V., Troshin, P., Troshina, O. & DuBuske, L. (2008). “Penetration of Fullerene C60 Derivatives through Biological Membranes,” Fullerenes Nanotubes and Carbon Nanostructures, 16 (2) 89-102.
Publisher – Google Scholar
Badamshina, E. R. & Gafurova, M. P. (2008). “Characteristics of Fullerene C60-Doped Polymers,” Polymer Science. Series B, 50 (7-8) 215—225.
Publisher – Google Scholar
Da Ros, T. (2008). “Twenty Years of Promises: Fullerene in Medicinal Chemistry,” Carbon Materials: Chemistry and Physics. V.1. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, Cataldo, F., and Da Ros, T. (eds), Springer.
Publisher – Google Scholar
Lyon, D. Y., Adams, L. K., Falkner, J. C. & Alvarez, P. J. (2006). “Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size,” Environmental Science & Technology, 40 (14) 4360-4366.
Publisher – Google Scholar
Minoru, I. (1978). “Studies on Lipoperoxide of Normal Pregnant Women and Patient Toxemia of Pregnancy,” Clinica Chimica Acta, 84 (1-2) 1-9.
Publisher – Google Scholar
Okovitiy, S. V. (2003). ‘Clinical Pharmacology of Antioxidants,’ FARMindex: Praktik, 5, 85-111 [in Russian].
Piotrovskiy, L. B., Eropkin, M. Y., Eropkina, E. M., Dumpis, M. A. & Kiselev, O. I. (2007). ‘Mechanisms of Biological Activity of Fullerenes — Relation to Aggregate State,’ Psychopharmacology @ Biological Narcology, 7 (2) 1548-1554 [in Russian].
Promyslov, Sh. M. & Demchuk, M. L. (1990). ‘A Modified Procedure for Estimation of Total Antioxidant Activity of Blood Serum,’ Voprosy Meditsinskoi Khimii, 36 (4) 90-92 [in Russian].
Google Scholar
Weng, D., Lee, H. K., Levon, K., Mao, J., Scrivens, W. A., Stephens, E. B. & Tour, J. M. (1999). “The Influence of Buckminsterfullerenes and their Derivatives on Polymer Properties,” European Polymer Journal, 35 (5) 867-878.
Publisher – Google Scholar
Yevlampieva, N. P., Dmitrieva, T. S., Melenevskaya, E. Yu. Zaitseva, I. I.& Ryumtsev, E. I. (2007). “Interaction of Polystyrene and Fullerene C60 in Benzene: Composition and Molecular Properties of the Product,” Polymer Science. Series A, 49 (3) 284-291.
Publisher – Google Scholar
Zhou, X., Liu, J., Jin, Z., Gu, Z., Wu, Y. & Sun, Y. (1997). “Solubility of Fullerene C60 and C70 in Toluene, o-Xylene and Carbon Disulfide at Various Temperatures,” Fullerene Science and Technology, 5 (1) 285-290.
Publisher – Google Scholar