Introduction
The word “apoptosis” is derived from the ancient Greek word meaning the falling off of petals from a flower or of leaves from a tree in autumn. The term was first introduced by Kerr, Wyllie and Currie in 1972, to describe the morphological processes leading to controlled cellular self-destruction. As apoptosis was introduced as a term describing a specific morphology of cell death, it should not be used synonymously with the term “programmed cell death (PCD)”, which usually occurs via apoptosis. (Lawson A 2003) The apoptotic mode of cell death plays an important role in the development, regulation and maintenance of the cell populations in both physiological and pathological conditions.
How Apoptosis is Different from Necrosis?
In apoptosis, cell is an active participant in its own demise. This type of cell death is controlled, energy dependent and can affect individual or cluster of cells. In contrast, “Necrosis” is considered to be toxic process where the cell is a passive victim, follows an energy-independent mode of death and usually affects large field of cells. (Elmore S 2000) Although apoptosis and necrosis differ in their mechanism, there are evidences indicating that they both represent morphologic expression of shared biochemical network known as “Apoptosis-Necrosis Continum”. (Zeiss CJ 2003).
Table 1: Showing Difference between Apoptosis and Necrosis
(Elmore S 2000, Roche] (Modified)
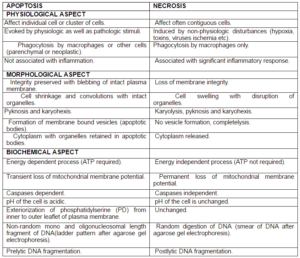
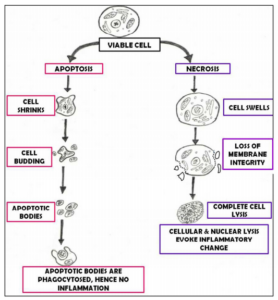
Figure 1: Showing Difference between Apoptosis and Necrosis
Apoptosis in Biological Process– Apoptosis plays dual role in biological process:
I. Physiologic apoptosis is implicated in the following : (Singh N 2007, Rastogi R et al 2009).
- Embryogenesis including implantation and organogenesis.
- Development and involution of various organ system eg: Nervous system, Immune System and Regression of mullerian and wolfian duct.
- Initiation of menstruation in adult female.
- Autoregulatory mechanism of intestine eg: maintainence of iron absorption.
- Normal placental development.
- Production of erythrocytes via erthropoiesis.
- Various infections eg: certain types of viral hepatitis, where apoptosis is needed to destroy pathogen invaded cells.
- In wound healing, apoptosis is involved in removal of inflammatory cells and evolution of granulation tissue into scar tissue.
- Remodelling of adult tissue eg: Follicular atresia of post-ovulatory follicle, post-weaning mammary gland involution.
- Elimination of activated or autoregressive immune cells either due to maturation in central lymphoid organs (bone marrow or thymus) or in peripheral tissue.
- Age induced apoptosis:
With the process of ageing, some of the cells die more rapidly as a result of apoptosis. This is because of generated oxidative stress as a consequence of accumulated free radical damage to mitochondrial DNA and hence known as age induced apoptosis. (Elmore S 2000, Harman D 1992).
12.Apoptosis in oral tissues:
During oral embryogenesis, regulation of delicate balance between cell death and cell survival and epithelial-mesenchymal interaction play an essential role in determining which cells to be shed and which one to survive. Epithelial cells require contact with each other for survival signals. Detachment of epithelial cells from neighbouring cells triggers apoptosis. This type of apoptosis induced by loss of adhesion of cells known as “Anoikis”. (Loro LL et al 2005).
II. Pathologic apoptosis involves: (Singh N 2007, Rastogi R et al 2009, Loro LL et al 2005).
- Inadequate apoptosis/Downregulated: includes-
- Cancer (Oral, colorectal, Hepatic, Prostrate, Leukemia, Neuroblastoma).
- Autoimmune disorder (SLE, Myasthenia gravis, Autoimmune proliferative syndrome, Sjogren syndrome).
- Infections (viral infections).
2.Extreme Apoptosis/Upregulateds: includes-
- Neurodegenerative disorder: Alzheimers diseases, Parkinsonism, Hutingtons chorea, Stroke, Brain truma, Spinal cord injury, Amylotropic lateral sclerosis, Retinitis pigmentosa, Epilepsy etc.
- Cardiovascular disorders: Heart failure, Myocardial infarction, Stroke.
- Hematologic disorders: Aplastic anemia, Myelodysplatic syndrome, T CD4 + lymphocytopenia.
- Others: Inflammation, Sepsis, Diabetes, Alopecia, AIDS, Polycystic ovarian diseases.
Stages of Apoptosis
The process of apoptosis is divided into 3 process:
I. Phase of Initiation: In this phase, cell receives various intra and intercellular signals via intrinsic and extrinsic pathway resulting in apoptotic death sequence.
Apoptotic inductors can be physiological (hormones, cytokines etc), biological (bacteria, viruses, parasites etc), chemical (medication) or physical (radiations, toxins etc). In addition, one stimulus can generate different and even opposing effects in different cell types and in different development stages of same cell type. (Torres L et al 2003, Thompson CB, 1995).
II. Phase of Execution: Once the cell has received the signal to induce apoptosis, it loses contact with neighbouring cells and undergoes characteristic morphologic and biochemical alterations. (Torres L et al 2003).
III. Phase of Elimination: In this phase, remnants of cells undergoing apoptosis are quickly and efficiently eliminated by professional phagocytes or non-professional phagocytes such as dendritic cells, epithelial cells and fibroblasts therefore not associated with inflammation. (Torres L et al 2003, Gregory CD 2000).
Morphological Alterations in Apoptosis
- Nuclear alterations: Morphological hallmark of apoptosis in nucleus are chromatin condensation & nuclear fragmentation which can be seen with light microscopy, electron microscopy, fluorescence microscopy and also by using labelled dUTP by the enzyme terminal deoxynucleotide transferase nick end (TUNEL) method. Structural protein processed by caspases associated with apoptotic morphology includes actin, spectrin, gelsolin, β-catenin, Laminin A & B, Keratin 18 & 19 etc. (Chamond RR et al 1999, Saraste A et al 2000, Ziegler U et al 2004).
- Alteration in Cell membrane & Cytosol: Initially during apoptosis, cell detaches from its substratum & adjacent cells. Membrane & organelles are well preserved. Subsequently cells start to show extensions or protrubrences of plasma membrane commonly referred to as blebs. Following cell shrinkage, these blebs separate forming apoptotic bodies, which are round, smooth membrane bound remnants densely packed with organelles & nuclear fragments. (Elmore S 2000, Chamond RR et al 1999, Saraste A et al 2000, Ziegler U et al 2004) Apoptotic bodies are rapidly phagocytosed by neighbouring cells including macrophages & parenchymal cells hence there is essentially no inflammatory reaction associated with process of apoptosis.
- Mitochondrial alterations: Mitochondrial membrane permeabilisation has a central role during apoptosis degradation cascade. Proapoptotic members of Bcl-2 family are involved in initiation of mitochondrial membrane permeabilisation where as antiapoptotic factors of Bcl-2 family inhibits this process. Moreover there is loss of transmembrane potential. (Chamond RR et al 1999, Ziegler U et al 2004).
Microscopy of Apoptotic Cells: In light microscopy with hematoxylin and eosin stain, an apoptotic cell appears as a small round or oval mass, having dark eosinophilic cytoplasm and dense purple nuclear chromatin fragments.
Ultrastructurally, an apoptotic cell will show characterstic peripheral aggregation of electron-dense nuclear material but there can also be uniformly dense nuclei. (Gobe G et al 2008).
Biochemical Alterations in Apoptosis:
- Internucleosomal DNA Fragmentation: It is a characterstic form of DNA degradation which occurs by activation of endogenous DNAase in which genome is cleaved at nucleosomal sites, generating a “ladder” of DNA fragments when analysed by agarose gel electrophoresis and by TUNEL assay. (Saraste A et al 2000, Ziegler U et al 2004, Compton MM 1992, Nagata S. 2000).
- Cytoplasmic Acidification: Acidification as a concomitant of cell death was first reported by Nedergard in case of neuronal injury and subsequently by Burry and Eastman who recognised acidification to be a feature of apoptosis. (Gottlieb RA. 1996) It has been suggested that a drop of cytoplasmic pH will have a profound effect on activity of enzyme like DNAase II, protease, transglutaminase and sphingomyelinase. (Barry MA et al 1993, Meisenholder GW et al 1996, Melino G et al 1994, Kanfer JN et al 1994).
- Externalisation of Phosphatidylserine (PD): In normal cells, PD is present on inner leaflet of plasma membrane but in apoptotic cell, this phospholipid “flips” out and is exposed on the outer layer membrane, where it is recognised by macrophages. (Ziegler U et al 2004, Savill J 2000).
- Other Biochemical Feature Includes: Caspases interaction, Loss of mitochondrial membrane potential, Increase of free ionic calcium, Proteolysis of laminin B. (Chamond RR et al 1999, Saraste A et al 2000, Ziegler U et al 2004).
Apoptosis Signalling Cascade
Major Players in Apoptosis Includes:
- CASPASES: The term caspases is derived from cysteine-dependent aspartate-specific proteases. The caspases are family of cysteine proteases homologous to C. elegans ced-3 and are of central importance in the apoptotic signalling network which are activated in most cases of apoptotic cell death. (Lawson A 2003).
Classification of Caspases: Depending upon the length of amino terminal prodomain. (Rastogi R et al 2009
Initiator (Apical): Caspase-2, Caspase-8, Caspase-9, Caspase-10
Effector (Executioner): Caspase-3, Caspase-6, Caspase-7
2. Bcl-2 Family Proteins: The member of Bcl-2 family are a group of crucial regulatory factors in apoptosis. Bcl-2 proteins are characterised by presence of conserved sequence motifs known as Bcl-2 homology (BH) domains. 4 domains have been described- BH1, BH2, BH3, BH4. [Singh N 2007, Strasser A et al 2000, Fan JJ et al 2005, Paula C et al 2003).
Table 2: Showing Classification of Bcl-2 Family Proteins: Depending upon their Function Domain Present
(Fan JJ et al 2005, Paula C et al 2003)

3.Tumor Necrosis Factor Receptor Superfamily: Extrinsic apoptosis signalling is mediated by the activation of so called “death receptors” (DRs). These cell surface DRs belongs to Tumor necrosis factor receptor (TNFR) superfamily. They transmit their apoptotic signals following binding with specific ligands. (Lawson A 2003, Strasser A et al 2000) The best characterised family member in humans are- TNF-R1, Fas (Apo1 or CD95), DR-3 (Apo3, WSL-1, TRAMP or LARD), DR-4/ TRAIL-R1, DR-5 (TRAIL-R2, Apo-2, TRICK2 or KILLER), DR-6 and NGF-R. (Paula C et al 2003, French LE et al 2003) .
4. Adaptor Molecules: Adaptor proteins are the link between cell death initiator i.e caspases and the cell death regulator i.e death receptors and Bcl-2 family members. These links take form physical association between TNFR family members on one side and caspases on other allowing caspases aggregation and activation. Examples of adaptor proteins includes: Fas-associating death domain protein/mediator of receptor-induced toxicity (FADD/MORT1), TNF-R1-associated death domain protein (TRADD), and receptor-interacting protein (RIP). (Strasser A et al 2000).
Molecular Mechanism of Apoptosis– Apoptosis is a tightly regulated and highly efficient cell death program which requires interplay of multitude of factors.
Types of Apoptotic Mechanism:
- Via stimuli/signals arising outside or inside the cell-
- Extrinsic (Death receptor activated) or Intrinsic (mitochondria activated).
- Caspases dependent or Caspases independent.
- p53 dependent or p53 independent.
2.Via reactive oxygen species (ROS)
Apoptosis may be activated by variety of stimuli including Ionising radiations anticancer drug, Heat, ultraviolet light, oxygen free radicals, hydrogen peroxide etc. (Kam P et al 2000).
Extrinsic/ Death Receptor Activated Pathway – Involves the initiation of apoptosis through ligation of plasma membrane death receptors therefore also known as Death receptor pathway resulting in the recruitment of adaptor proteins. Caspase 8, 10, 2 are the caspases involved in the death receptor pathway which needs to be activated. (Fan JJ et al 2005, Paula C et al 2003) Types of signalling involved in extrinsic apoptosis are Fas L Signalling, TNF Signalling, Apo 3L and Apo 6L Signalling & TRAIL Signalling. (Paula C et al 2003).
Intrinsic/ Mitochondrial Cell Death Pathway — Also known as Mitochondrial cell death pathway since apoptosis occurs secondary to imbalance in intracellular homeostasis. (Fan JJ et al 2005, Paula C et al 2003). Following an apoptotic trigger, several apoptogenic proteins are released from mitochondria. (Hail N et al 2006) Chief molecule involved in execution of this pathway is cytochrome c which is is released into cytoplasm and interacts with apoptotic proteases activating factor-1 (Apaf-1), dATP/ATP and procaspase-9 resulting in formation of massive complex known as apotosome. Other mitochondrial proapoptotic factor are Second mitochondrial activator of caspases/ Direct IAP binding protein of low isoelectric point (SMAC/DIABLO) and apoptosis inducing factor (AIF). (Lawson A 2003, Paula C et al 2003).
Endoplasmic reticulum (ER) has also been implicated in process of apoptosis. The characterstic mediator of apoptosis under ER stress is caspase 12. ER stress is particularly caused by accumulation of unfolded and misfolded proteins in ER lumen and/or the perturbation of calcium ion homeostasis. (Fan JJ et al 2005, Morishima N et al 2002).
Common Pathway: Once activated either via extrinsic or intrinsic pathway, apoptosis activator caspase such as caspase 2, 8, 9, 10 will activate other downstream apoptosis executioner caspases including caspase 3, 6, 7 which will further activate other proteins. (Fan JJ et al 2005).
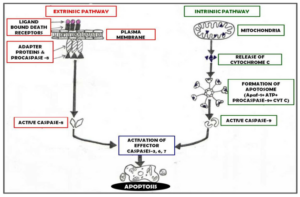
Figure 2: Showing Pathways of Apoptosis Mechanism
Non-Caspase Dependent Mechanisms (Hail N et al 2006)
- Via Non-caspase proteases like cathepsins, calpain and granzyme and Omi/HtrA2.
- Via ex-mitochondrial proteins like Apoptosis Inducing Factor (AIF), Mitochondrial Endonuclease G, WOX1/WWOX/FOR, AIF Homologous Mitochondrian Associated Inducer of Death (AMID) and Cytochrome c.
Apoptosis Induction via Reactive Oxygen Species (ROS)
ROS and mitochondria play an important role in apoptosis under both physiologic and pathologic conditions. ROS includes free radical species such as hydroxyl (OH), alkoxyl (RO) or peroxyl (ROO-) superoxide (O2-) or nitroxyl radical ( NO-) and non-radical hydrogen peroxide (H2O2), organic hydroperoxides (ROOH) and hypocholorous acid (HOCL). (Simon HU et al 2000) ROS are generated by inflammatory cells which accumulate in both allergic (French LE et al 2003, Simon HU 1997) and non allergic (Simon HU 2000, Dibbert B et al 1999) inflammation. ROS can induce apoptosis in many different cell system. For eg: H2O2 induced apoptosis in neutrophil which can be prevented by catalase. (Kasahara Y et al 1997) ROS can induce apoptosis either via Death receptor mediated apoptosis or through mitochondria, Endoplasmic reticulum and calcium. (Hail N et al 2006, Simon HU et al 2000).
Regulation of Apoptosis
Apoptosis is a controlled genetically programmed event which gets activated when a cell encounters a specific death inducing signal or death stimulus. All cells of a multicellular animal might undergo systematic self destruction unless cell death is constantly inhibited by survival signals as provided by other cells e.g. growth factors, hormones, nutrients. These survival signals control apoptosis by maintaining an equilibrium between pro-apoptotic and antiapoptotic regulatory molecules. Certain molecules, acting as modulators of apoptosis are- Bcl-2 Family, Voltage Dependent Anion Channel (VDAC)/Porins, Adenine Nucleotide Translocator (ANT), Inhibitor of Apoptotic Proteins (IAP), FLIP Proteins, Cytotoxic T-Cells, PI-3 Kinase Pathway. (Brunelle JK et al 2002, Chen M et al 2002, Mayer B et al 2003, Boise LH et al 1997).
Clinical Implication of Apoptosis in Oral Health and Diseases:
Proper function of the apoptotic machinery is of fundamental importance during the growth and development of the organism, because apoptosis in accord with cell division ensures the proper shaping and the structural and functional integrity of the various tissues and organs. (Nikitakis N G et al 2004, Matalova E et al 2004) During oral embryogenesis, regulation of the delicate balance between cell death and cell survival and epithelial-mesenchymal interaction play an essential role in determining which cell to be shed and which one to survive. Selected cells are destined to die by apoptosis termed as PCD (Programmed cell death) (Loro LL et al 2005).
Apoptosis plays an important role during tooth morphogenesis. Teeth are example of epithelial mesenchymal organs and are often used as model for studying the nature of such interactions and signalling controlling morphogenesis, histogenesis and cytodifferentiation. (Kim JY et al 2006, Lesot H et al 1996, Vaahtokari A et al 1996) During tooth development apoptosis found to occur in dental lamina, enamel organ, enamel knot, ameloblasts and odontoblasts and periodontal tissues. (Matalova E et al 2004, Lucas H et al 2010).
A dysfunctional apoptotic system can lead to either excessive removal or prolonged survival of cells. Therefore, dysregulation of apoptosis is involved in the pathogenesis of a variety of diseases like reactive oral lesions, (Jafarzadeh H et al 2006) recurrent apthous ulceration, (Honma T et al 1985) periodontal lesions, (Jarnbring F et al 2002) mucocutaneous lesions (erythema multiforme, lupus erythematosus, pemphigus vulgaris, epidermolysis bullosa, lichen planus,) (Chrysomali E et al 1997, Vaishnaw AK et al 1999, Puviani M et al 2003, Yoneda K 2001, Murrah VA et al 2006) viral infections, (Chamond RR et al 1999) candidal infection, (Villar CC et al 2010, Rouabhia M et al 2012) sjogren syndrome, (Manganelli P et al 2003) salivary gland tumors, (Jia L et al 2004) odontogenic cysts, (Artese L et al 2008) odontogenic tumors, (Kumamoto H et al 2005) leukoplakia (Tanda N et al 2000) and Oral squamous cell carcinoma. (Kaufmann SH et al 2000).
Conclusion
Apoptosis is regarded as a vigilantly regulated active process, being initiated by various physiologic and pathologic stimuli, characterized by specific morphological and biochemical alterations mediated by several molecules and regulated by balance between proapoptotic and antiapoptotic signals. The importance of understanding the mechanism of apoptosis is crucial because it has got a vital role in both health and disease. Moreover, the pervasive association of apoptosis in the pathobiology of disease lends itself to therapeutic intervention at many different checkpoints.
References
Artese, L., Lezzi, G., Piattelli, A., Rubini, C., Goteri, G., Pernotti, V., Piccirilli, M. & Carinci, F. (2008). “p16 Expression in Odontogenic Cysts,” Dental Research Journal, 5 (2) 61-64.
Publisher
Ashe, P. C. & Berry, M. D. (2003). “Apoptotic Signaling Cascades,” Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27, 199— 214.
Publisher – Google Scholar
Barry, M. A. & Eastman, A. (1993). “Identification of Deoxyribonuclease II as an Endonuclease Involved in Apoptosis,”Archives of Biochemistry and Biophysics, 300 (1) 440-450.
Publisher – Google Scholar
Brunelle, J. K. & Chandel, N. S. (2002). “Oxygen Deprivation Induced Cell Death: An Update,” Apoptosis, 7 (6) 475—482.
Publisher – Google Scholar
Boise, L. H. & Thompson, C. B. (1997). “Bcl-xL Can Inhibit Apoptosis in Cells that Have Undergone Fas-Induced Protease Activation,” Proceedings of The National Academy of Sciences of the USA, 94 (8) 3759-3764.
Publisher – Google Scholar
Chamond, R. R., Anon, J. C., Aguilar, C. M. & Pasadas, G. (1999). “Apoptosis and Disease,” Alergologia E Inmunologia Clinica, 14 (6) 367-374.
Publisher
Chen, M. & Wang, J. (2002). “Initiator Caspases in Apoptosis Signaling Pathways,” Apoptosis, 7 (4) 313—319.
Publisher – Google Scholar
Chrysomali, E., Nur, F. L., Dekker, N. P., Papanicolaou, S. I. & Regezi, J. A. (1997). “Apoptosis in Oral Erythema Multiforme,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 83 (2) 272-80.
Publisher – Google Scholar
Compton, M. M. (1992). “A Biochemical Hallmark of Apoptosis: Internucleosomal Degradation of the Genome,” Cancer and Metastasis Reviews, 11 (2) 105-119.
Publisher – Google Scholar
Dibbert, B., Weber, M., Nikolaizik, W. H. et al. (1999). “Cytokine-mediated Bax Deficiency and Consequent Delayed Neutrophil Apoptosis: A General Mechanism to Accumulate Effector Cells in Inflammation,” Proceedings of The National Academy of Sciences of the USA, 96 (23) 13330— 13335.
Publisher – Google Scholar
Elmore, S. (2000). “Apoptosis: A Review of Programmed Cell Death,” Toxicologic Pathology, 35 (4) 495-51.
Publisher – Google Scholar
Fan, T. J., Han, L. H., Cong, R. S. & Liang. J. (2005). “Caspase Family Proteases and Apoptosis,” Acta Biochimica et Biophysica Sinica, 37 (11) 719—727.
Publisher – Google Scholar
French, L. E. & Tschopp, J. (2003). “Protein-Based Therapeutic Approaches Targeting Death Receptors,” Cell Death and Differentiation, 10 (1) 117-23.
Publisher – Google Scholar
Gobe, G. & Harmon, B. (15 Dec 2008). “Apoptosis: Morphological Criteria and Other Assays,” [OLINE] ELS, John Wiley & Sons. 10 July 2012. http://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0002569.pub3/full
Publisher
Gottlieb, R. A. (1996). “Cell Acidification in Apoptosis,” Apoptosis, 1(1) 40-48.
Publisher – Google Scholar
Gregory, C. D. (2000). “CD14-Dependent Clearance of Apoptotic Cells: Relevance to Immune System,” Current Opinion in Immunology, 12 (1) 27-34.
Publisher – Google Scholar
Hai, l. N., Carter, B. Z., Konopleva, M. & Andreeff, M. (2006). “Apoptosis Effector Mechanisms: A Requiem Performed in Different Keys,” Apoptosis, 11(6) 889—904.
Publisher – Google Scholar
Harman, D. (1992). “Role of Free Radicals in Aging and Disease,” Annals of the New York Academy of Sciences, 673, 126—41.
Publisher – Google Scholar
Honma, T., Saito, T. & Fujioka, Y. (1985). “Possible Role of Apoptotic Cells of the Oral Epithelium in the Pathogenesis of Aphthous Ulceration,” Oral Surgery, Oral Medicine, Oral Pathology, 59 (4) 379—387.
Publisher – Google Scholar
Jafarzadeh, H., Sanatkhani, M. & Mohtasham, N. (2006). “Oral Pyogenic Granuloma: A Review,” Journal of Oral Science, 48 (4) 167-175.
Publisher – Google Scholar
Jarnbring, F., Somogyi, E., Dalton, J., Gustafsson, A. & Klinge, B. (2002). “Quantitative Assessment of Apoptotic and Proliferative Gingival Keratinocytes in Oral and Sulcular Epithelium in Patients with Gingivitis and Periodontitis,” Journal of Clinical Periodontology, 29 (12) 1065—1071.
Publisher – Google Scholar
Jia, L., Esguerra, R. L., Tang, X. et al. (2004). “Prognostic Value of Apoptosis and Apoptosis-Associated Proteins in Salivary Gland Adenoid Cystic Carcinoma,” Pathology International, 54 (4) 217-23.
Publisher – Google Scholar
Kam, P. C. A. & Ferch, N. I. (2000). “Apoptosis: Mechanisms and Clinical Implications,” Anaesthesia, 55 (11) 1081-1093.
Publisher – Google Scholar
Kanfer, J. N., Young, O. M. & Shapiro, D. (1995). ‘The Metabolism of Sphingomyelin. Purification and Properties of a Sphingomyelin- Cleaving Enzyme from Rat Liver Tissue,‘ The Journal of Biological Chemistry, 241(5) 1081-1084.
Kasahara, Y., Kazuyuki, I., Yachie, A. et. al. (1997). “Involvement of Reactive Oxygen Intermediates in Spontaneous and CD95 (Fas/APO-1)-Mediated Apoptosis of Neutrophils,” Blood, 89 (5) 1748—1753.
Publisher – Google Scholar
Kaufmann, S. H. & Gores, G. J. (2000). “Apoptosis in Cancer: Cause and Cure,” BioEssays, 22 (11) 1007- 1017.
Publisher – Google Scholar
Kim, J. Y., Cha, Y. G., Cho, S. W. et al. (2006). “Inhibition of Apoptosis in Early Tooth Development Alters Tooth Shape and Size,” Journal of Dental Research, 85 (6) 530-535.
Publisher – Google Scholar
Kumamoto, H. & Ooya, K. (2005). “Detection of Mitochondria-Mediated Apoptosis Signalling Molecules in Ameloblastomas,” Journal of Oral Pathology & Medicine, 34 (9) 565—72.
Publisher – Google Scholar
Lawen, A. (2003). “Apoptosis–An Introduction,” Bio Essays, 25 (9) 888—896.
Publisher – Google Scholar
Lesot, H., Vonesch, J. L., Peterka, M. et al. (1996). “Mouse Molar Morphogenesis Revisited by Three Dimensional Reconstruction. II. Spatial Distribution of Mitoses and Apoptosis in Cap to Bell Staged First and Second Upper Molar Teeth,” The International Journal of Developmental Biology, 40(5) 1017-1031.
Publisher – Google Scholar
Loro, L. L., Vintermyr, O. K. & Johannessen, A. C. (2005). “Apoptosis in Normal and Diseased Tissue,” Oral Diseases, 11(5) 274-287.
Publisher – Google Scholar
Lucas, H., Bartold, P. M. , Dharmapatni, A. A. S. S. K. Holding, C. A. & Haynes, D. R. (2010). “Inhibition of Apoptosis in Periodontitis,” Journal of Dental Research, 89 (1) 29-33.
Publisher – Google Scholar
Manganelli, P. & Fietta, P. (2003). “Apoptosis and Sjögren Syndrome,” Seminars in Arthritis and Rheumatism, 33 (1) 49-65.
Publisher – Google Scholar
Matalova, E., Tucker, A. S. & Sharpe, P. T. (2004). “Death in the Life of a Tooth,” Journal of Dental Research, 83(1) 11-16.
Publisher – Google Scholar
Mayer, B. & Oberbauer, R. (2003). “Mitochondrial Regulation of Apoptosis,” News in Physiological Sciences, 18, 89- 94.
Publisher – Google Scholar
Meisenholder, G. W., Martin, S. J., Green, D. R. et al. (1996). “Events in Apoptosis: Acidification is Downstream of Protease Activation and Bcl-2 Protection,” The Journal of Biological Chemistry, 271(27) 16260—16262.
Publisher – Google Scholar
Melino, G., Annicchiarico-Petruzzelli, M., Piredda, L. et al. (1994). “Tissue Transglutaminase and Apoptosis: Sense and Antisense Transfection Studies with Human Neuroblastoma Cells,” Molecular and Cellular Biology, 14 (10) 6584-6596.
Publisher – Google Scholar
Morishima, N., Nakanishi, K., Takenouchi, H., Shibata, T. & Yasuhiko, Y. (2002). “An Endoplasmic Reticulum Stress-Specific Caspase Cascade in Apoptosis. Cytochrome C-Independent Activation of Caspase-9 by Caspase-12,” The Journal of Biological Chemistry, 277, 34287−34294.
Publisher – Google Scholar
Murrah, V. A. & Gilchrist, E. P.(1996). “Assessment of Apoptosis in Oral Lichen Planus,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 82 (2) 209-210.
Publisher – Google Scholar
Nagata, S. (2000). “Apoptotic DNA Fragmentation,” Experimental Cell Research 256 (1) 12-8.
Publisher – Google Scholar
Nikitakis, N. G., Sauk, J. J. & Papanicolaou, S. I. (2004). “The Role of Apoptosis in Oral Disease: Mechanisms; Aberrations in Neoplastic, Autoimmune, Infectious, Hematologic, and Developmental Diseases; and Therapeutic Opportunities,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 97 (4) 476-90.
Publisher – Google Scholar
Puviani, M., Marconi, A., Cozzani, E. & Pincelli, C. (2003). “Fas Ligand in Pemphigus Sera Induces Keratinocyte Apoptosis through the Activation of Caspase-8,” The Journal of Investigative Dermatology, 120 (1) 164-7.
Publisher – Google Scholar
Rastogi, R., Richa, P. & Sinha, R. (2009). ‘Apoptosis: Molecular Mechanism and Pathogenecity,’ EXCLI Journal, 8, 151-181.
Roche. ‘Apoptosis, Cell Death and Cell Proliferation Manual,’ Roche applied sciences. (3rd edition), Manheim, Germany.
Rouabhia, M., Semlali, A., Chandra, J. et al. (2012). “Disruption of the ECM33 Gene in Candida Albicans Prevents Biofilm Formation, Engineered Human Oral Mucosa Tissue Damage and Gingival Cell Necrosis/Apoptosis,” Mediators of Inflammation, 2012, 9 pages.
Publisher – Google Scholar
Saraste, A. & Pulkki, K. (2000). “Morphologic and Biochemical Hallmarks of Apoptosis,” Cardiovascular Research, 45, 528—537.
Publisher – Google Scholar
Savill, J. & Fadok, V. (2000). “Corpse Clearance Defines the Meaning of Cell Death,” NATURE, 407(6805) 784-788.
Publisher – Google Scholar
Simon, H. U., Yehia, A. H. & Schaffer, F. L. (2000). “Role of Reactive Oxygen Species (ROS) in Apoptosis Induction,”Apoptosis, 5 (5) 415—418.
Publisher – Google Scholar
Simon, H. U., Yousefi, S., Schranz, C., Schapowal, A., Bachert, C. & Blaser, K. (1997). “Direct Demonstration of Delayed Eosinophil Apoptosis as a Mechanism Causing Tissue Eosinophilia,” The Journal of Immunology, 158 (8) 3902—8.
Publisher – Google Scholar
Singh, N. (2007). ‘Apoptosis in Health and Diseases and Modulation of Apoptosis for Therapy: An Overview,’ Indian Journal of clinical Biochemistry, 22 (2) 6-16.
Strasser, A., Connor, L. &. Dixit, V. M. (2000). “Apoptosis Signaling,” Annual Review of Biochemistry, 69, 217—45.
Publisher – Google Scholar
Tanda, N., Mori, S., Saito, K., Ikawa, K. & Sakamoto, S. (2000). “Expression of Apoptotic Signalling Proteins in Leukoplakia and Oral Lichen Planus: Quantitative and Topographical Studies,” Journal of Oral Pathology & Medicine, 29 (8) 385-93.
Publisher – Google Scholar
Thompson, C. B. (1995). ‘Apoptosis in Pathogenesis and Treatment of Diseases,’ Science, 267, 1456-1462.
Torres, L. & Varges, F. (2003). ‘Apoptosis: The Phenomena and its Determination,’ Tec Pecu Max, 41 (1) 49-62.
Vaahtokari, A., Aberg, T. & Thesleff, I. (1996). “Apoptosis in Developing Tooth: Association with an Embryonic Signalling Center and Suppression of EGF and FGF-4,” Development, 122, 121-129.
Publisher
Vaishnaw, A. K., Toubi, E., Ohsako, S. et al. (1999). “The Spectrum of Apoptotic Defects and Clinical Manifestations, including Systemic Lupus Erythematosus, in Humans with Cd95 (Fas/Apo-1) Mutations,” Arthritis & Rheumatism, 42 (9) 1833—1842.
Publisher – Google Scholar
Villar, C. C. & Zhao, X. R. (2010). “Candida Albicans Induces Early Apoptosis Followed by Secondary Necrosis in Oral Epithelial Cells,” Molecular Oral Microbiology, 25 (3) 215-25.
Publisher – Google Scholar
Yoneda, K., Furukawa, T., Zheng, Y.- J. et al. (2004). “An Autocrine/Paracrine Loop Linking Keratin 14 Aggregates to Tumor Necrosis Factor-α Mediated Cytotoxicity in a Keratinocyte Model of Epidermolysis Bullosa Simplex,” The Journal of Biological Chemistry, 279 (8) 7296—7303.
Publisher – Google Scholar
Zeiss, C. J. (2003). “The Apoptosis-Necrosis Continuum: Insight from Genetically Altered Mice,” Veterinary Pathology, 40 (5) 481-495.
Publisher – Google Scholar
Ziegler, U. & Groscurth P. (2004). “Morphological Features of Cell Death,” News in Physiological Sciences, 19, 124-128.
Publisher – Google Scholar






