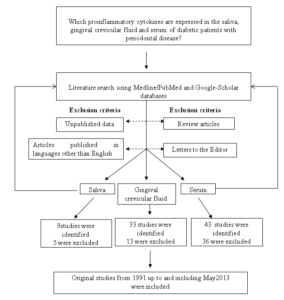
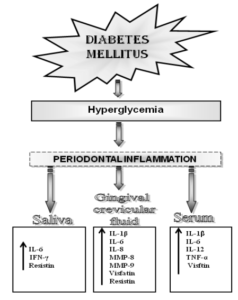
Argiles, J. M., Lopez-Soriano, J. & Lopez-Soriano, F. J. (1994). “Cytokines and Diabetes: The Final Step? Involvement of TNF-alpha in Both Type I and II Diabetes Mellitus,” Hormone and Metabolic Research , 26(10), 447-9.
Publisher – Google Scholar
Bandyopadhyay, D., Marlow, N. M., Fernandes, J. K. & Leite, R. S. (2010). “Periodontal Disease Progression and Glycaemic Control among Gullah African Americans with Type-2 Diabetes,” Journal of Clinical Periodontology, 37(6), 501-9.
Publisher – Google Scholar
Bartold, P. M. & Haynes, D. R. (1991). “Interleukin-6 Production by Human Gingival Fibroblasts,” Journal of Periodontal Research, 26(4), 339-45.
Publisher – Google Scholar
Chen, L., Wei, B., Li, J., Liu, F., Xuan, D., Xie, B. & Zhang, J. (2010). “Association of Periodontal Parameters with Metabolic Level and Systemic Inflammatory Markers in Patients with Type 2 Diabetes,” Journal of Periodontology, 81(3), 364-71.
Publisher – Google Scholar
Cole, C. M., Sundararaj, K. P., Leite, R. S., Nareika, A., Slate, E. H., Sanders, J. J., Lopes-Virella, M. F. & Huang, Y. (2008). “A Trend of Increase in Periodontal Interleukin-6 Expression across Patients with Neither Diabetes nor Periodontal Disease, Patients with Periodontal Disease Alone, and Patients with Both Diseases,” Journal of Periodontal Research, 43(6), 717-22.
Publisher – Google Scholar
Correa, F. O. B., Goncalves, D., Figueredo, C. M. S., Gustafsson, A. & Orrico, S. R. P. (2008). “The Short-Term Effectiveness of Non-Surgical Treatment in Reducing Levels of Interleukin-1beta and Proteases in Gingival Crevicular Fluid from Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis,” Journal of Periodontology, 79(11), 2143-50.
Publisher – Google Scholar
Costa, P. P., Trevisan, G. L., Macedo, G. O., Palioto, D. B., Souza, S. L. S., Grisi, M. F. M., Novaes, A. B., Jr. & Taba, M., Jr. (2010). “Salivary Interleukin-6, Matrix Metalloproteinase-8, and Osteoprotegerin in Patients with Periodontitis and Diabetes,” Journal of Periodontology, 81(3), 384-91.
Publisher – Google Scholar
Cutando, A., Gomez-Moreno, G., Villalba, J., Ferrera, M. J., Escames, G. & Acuna-Castroviejo, D. (2003). “Relationship between Salivary Melatonin Levels and Periodontal Status in Diabetic Patients,” Journal of Pineal Research, 35(4), 239-44.
Publisher – Google Scholar
Dag, A., Firat, E. T., Arikan, S., Kadiroglu, A. K. & Kaplan, A. (2009). “The Effect of Periodontal Therapy on Serum TNF-alpha and HbA1c Levels in Type 2 Diabetic Patients,” Australian Dental Journal, 54(1), 17-22.
Publisher – Google Scholar
Engebretson, S., Chertog, R., Nichols, A., Hey-Hadavi, J., Celenti, R. & Grbic, J. (2007). “Plasma Levels of Tumour Necrosis Factor-Alpha in Patients with Chronic Periodontitis and Type 2 Diabetes,” Journal of Clinical Periodontology, 34(1), 18-24.
Publisher – Google Scholar
Engebretson, S. P., Vossughi, F., Hey-Hadavi, J., Emingil, G. & Grbic, J. T. (2006). “The Influence of Diabetes on Gingival Crevicular Fluid Beta-Glucuronidase and Interleukin-8,” Journal of Clinical Periodontology, 33(11), 784-90.
Publisher – Google Scholar
Esposito, K., Nappo, F., Marfella, R., Giugliano, G., Giugliano, F., Ciotola, M., Quagliaro, L., Ceriello, A. & Giugliano, D. (2002). “Inflammatory Cytokine Concentrations are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress,” Circulation, 106(16), 2067-72.
Publisher – Google Scholar
Fitzsimmons, T. R., Sanders, A. E., Bartold, P. M. & Slade, G. D. (2010). “Local and Systemic Biomarkers in Gingival Crevicular Fluid Increase Odds of Periodontitis,” Journal of Clinical Periodontology, 37(1), 30-6.
Publisher – Google Scholar
Gemmell, E. & Seymour, G. J. (1994). “Modulation of Immune Responses to Periodontal Bacteria,” Current Opinion in Periodontology, 28-38.
Publisher – Google Scholar
Gimbrone, M. A., Jr., Cybulsky, M. I., Kume, N., Collins, T. & Resnick, N. (1995). “Vascular Endothelium. An Integrator of Pathophysiological Stimuli in Atherogenesis,” Annals of the New York Academy of Sciences, 748, 122-31; discussion 131-2.
Publisher – Google Scholar
Gomes, M. A. B., Rodrigues, F. H., Afonso-Cardoso, S. R., Buso, A. M., Silva, A. G., Favoreto, S., Jr. & Souza, M. A. (2006). “Levels of Immunoglobulin A1 and Messenger RNA for Interferon Gamma and Tumor Necrosis Factor Alpha in Total Saliva from Patients with Diabetes Mellitus Type 2 with Chronic Periodontal Disease,” Journal of Periodontal Research, 41(3), 177-83.
Publisher – Google Scholar
Grossi, S. G. & Genco, R. J. (1998). “Periodontal Disease and Diabetes Mellitus: A Two-Way Relationship,” Annals of Periodontology, 3(1), 51-61.
Publisher – Google Scholar
Hiroshima, Y., Bando, M., Inagaki, Y., Mihara, C., Kataoka, M., Murata, H., Shinohara, Y., Nagata, T. & Kido, J. (2012). “Resistin in Gingival Crevicular Fluid and Induction of Resistin Release by Porphyromonas Gingivalis Lipopolysaccharide in Human Neutrophils,” Journal of Periodontal Research, 47(5), 554-62.
Publisher – Google Scholar
Holloway, A. F., Rao, S. & Shannon, M. F. (2002). “Regulation of Cytokine Gene Transcription in the Immune System,”Molecular Immunology, 38(8), 567-80.
Publisher – Google Scholar
Ioannidou, E., Kao, D., Chang, N., Burleson, J. & Dongari-Bagtzoglou, A. (2006). “Elevated Serum Interleukin-6 (IL-6) in Solid-Organ Transplant Recipients is Positively Associated with Tissue Destruction and IL-6 Gene Expression in the Periodontium,” Journal of Periodontology, 77(11), 1871-8.
Publisher – Google Scholar
Javed, F., Al-Askar, M., Samaranayake, L. P. & Al-Hezaimi, K. (2013a). “Periodontal Disease in Habitual Cigarette Smokers and Nonsmokers with and Without Prediabetes,” American Journal of the Medical Sciences, 345(2), 94-8.
Publisher – Google Scholar
Javed, F., Klingspor, L., Sundin, U., Altamash, M., Klinge, B. & Engstrom, P. E. (2009). “Periodontal Conditions, Oral Candida Albicans and Salivary Proteins in Type 2 Diabetic Subjects with Emphasis on Gender,” BMC Oral Health, 9, 12.
Publisher – Google Scholar
Javed, F., Nasstrom, K., Benchimol, D., Altamash, M., Klinge, B. & Engstrom, P. E. (2007). “Comparison of Periodontal and Socioeconomic Status between Subjects with Type 2 Diabetes Mellitus and Non-Diabetic Controls,” Journal of Periodontology, 78(11), 2112-9.
Publisher – Google Scholar
Javed, F. & Romanos, G. E. (2009). “Impact of Diabetes Mellitus and Glycemic Control on the Osseointegration of Dental Implants: A Systematic Literature Review,” Journal of Periodontology, 80(11), 1719-30.
Publisher – Google Scholar
Javed, F., Thafeed Alghamdi, A. S., Mikami, T., Mehmood, A., Ahmed, H. B., Samaranayake, L. P. & Tenenbaum, H. C. (2013b). “Effect of Glycemic Control on Self-Perceived Oral Health, Periodontal Parameters and Alveolar Bone Loss among Patients with Prediabetes,” Journal of Periodontology.
Publisher – Google Scholar
Kardesler, L., Buduneli, N., Biyikoglu, B., Cetinkalp, S. & Kutukculer, N. (2008). “Gingival Crevicular Fluid PGE2, IL-1beta, t-PA, PAI-2 Levels in Type 2 Diabetes and Relationship with Periodontal Disease,” Clinical Biochemistry, 41(10-11), 863-8.
Publisher – Google Scholar
Kardesler, L., Buduneli, N., Cetinkalp, S. & Kinane, D. F. (2010). “Adipokines and Inflammatory Mediators after Initial Periodontal Treatment in Patients with Type 2 Diabetes and Chronic Periodontitis,” Journal of Periodontology, 81(1), 24-33.
Publisher – Google Scholar
Kinane, D. F. & Bartold, P. M. (2007). “Clinical Relevance of the Host Responses of Periodontitis,” Periodontology 2000, 43, 278-93.
Publisher – Google Scholar
Lalla, E., Kaplan, S., Chang, S.- M. J., Roth, G. A., Celenti, R., Hinckley, K., Greenberg, E. & Papapanou, P. N. (2006). “Periodontal Infection Profiles in Type 1 Diabetes,” Journal of Clinical Periodontology, 33(12), 855-62.
Publisher – Google Scholar
Lalla, E., Kaplan, S., Yang, J., Roth, G. A., Papapanou, P. N. & Greenberg, S. (2007). “Effects of Periodontal Therapy on Serum C-reactive Protein, sE-Selectin, and Tumor Necrosis Factor-Alpha Secretion by Peripheral Blood-Derived Macrophages in Diabetes. A Pilot Study,” Journal of Periodontal Research, 42(3), 274-82.
Publisher – Google Scholar
Mealey, B. L. & Ocampo, G. L. (2007). “Diabetes Mellitus and Periodontal Disease,” Periodontology 2000, 44, 127-53.
Publisher – Google Scholar
Nareika, A., Maldonado, A., He, L., Game, B. A., Slate, E. H., Sanders, J. J., London, S. D., Lopes-Virella, M. F. & Huang, Y. (2007). “High Glucose-Boosted Inflammatory Responses to Lipopolysaccharide are Suppressed by Statin,”Journal of Periodontal Research, 42(1), 31-8.
Publisher – Google Scholar
Navarro-Sanchez, A. B., Faria-Almeida, R. & Bascones-Martinez, A. (2007). “Effect of Non-Surgical Periodontal Therapy on Clinical and Immunological Response and Glycaemic Control in Type 2 Diabetic Patients with Moderate Periodontitis,”Journal of Clinical Periodontology, 34(10), 835-43.
Publisher – Google Scholar
Nishimura, F. & Murayama, Y. (2001). “Periodontal Inflammation and Insulin Resistance–Lessons from Obesity,” Journal of Dental Research, 80(8), 1690-4.
Publisher – Google Scholar
Nishimura, F., Taniguchi, A., Yamaguchi-Morimoto, M., Soga, Y., Iwamoto, Y., Kokeguchi, S., Kuroe, A., Fukushima, M., Nakai, Y. & Seino, Y. (2006). “Periodontal Infection and Dyslipidemia in Type 2 Diabetics: Association with Increased HMG-CoA Reductase Expression,” Hormone and Metabolic Research, 38(8), 530-5.
Publisher – Google Scholar
O’Connell, P. A. A., Taba, M., Nomizo, A., Foss Freitas, M. C., Suaid, F. A., Uyemura, S. A., Trevisan, G. L., Novaes, A. B., Souza, S. L., Palioto, D. B. & Grisi, M. F. (2008). “Effects of Periodontal Therapy on Glycemic Control and Inflammatory Markers,” Journal of Periodontology, 79(5), 774-83.
Publisher – Google Scholar
Pradeep, A. R., Raghavendra, N. M., Sharma, A., Patel, S. P., Raju, A., Kathariya, R., Rao, N. S. & Naik, S. B. (2012). “Association of Serum and Crevicular Visfatin Levels in Periodontal Health and Disease with Type 2 Diabetes Mellitus,”Journal of Periodontology, 83(5), 629-34.
Publisher – Google Scholar
Pradeep, A. R., Thorat Manojkumar, S., Garima, G. & Raju, A. (2010). “Serum Levels of Oncostatin M (a gp 130 Cytokine): An Inflammatory Biomarker in Periodontal Disease,” Biomarkers, 15(3), 277-82.
Publisher – Google Scholar
Salvi, G. E., Beck, J. D. & Offenbacher, S. (1998). “PGE2, IL-1 Beta, and TNF-Alpha Responses in Diabetics as Modifiers of Periodontal Disease Expression,” Annals of Periodontology, 3(1), 40-50.
Publisher – Google Scholar
Salvi, G. E., Collins, J. G., Yalda, B., Arnold, R. R., Lang, N. P. & Offenbacher, S. (1997a). “Monocytic TNF Alpha Secretion Patterns in IDDM Patients with Periodontal Diseases,” Journal of Clinical Periodontology, 24(1), 8-16.
Publisher – Google Scholar
Salvi, G. E., Franco, L. M., Braun, T. M., Lee, A., Rutger Persson, G., Lang, N. P. & Giannobile, W. V. (2010). “Pro-inflammatory Biomarkers during Experimental Gingivitis in Patients with Type 1 Diabetes Mellitus: A Proof-of-Concept Study,” Journal of Clinical Periodontology, 37(1), 9-16.
Publisher – Google Scholar
Salvi, G. E., Yalda, B., Collins, J. G., Jones, B. H., Smith, F. W., Arnold, R. R. & Offenbacher, S. (1997b). “Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients,”Journal of Periodontology, 68(2), 127-35.
Publisher – Google Scholar
Suh, K.- I., Kim, Y.- K. & Kho, H.- S. (2009). “Salivary Levels of IL-1beta, IL-6, IL-8, and TNF-Alpha in Patients with Burning Mouth Syndrome,” Archives of Oral Biology, 54(9), 797-802.
Publisher – Google Scholar