S100A8 and S100A9 belong to the S100 protein family, whose members contain 2 Ca²+ binding sites of the EF-hand type (Donato, 2001). S100A8 and S100A9 in this family make a heterodimeric complex called calprotectin, which is a pleiotropic protein associated with inflammation. Namely, calprotectin binds divalent cations, such as zinc ions and shows a bacteriostatic effect (Stříž and Trebichavský, 2004). Secondly, calprotectin is a mediator in the neutrophil recruitment in response to lipopolysaccharide (LPS) (Pouliot et al., 2008), and thereby the neutrophils migrate to the sites of inflammation via the circulation (Ryckman et al., 2003). Lastly, calprotectin has pro-inflammatory functions and exerts cytokine-like activities (Cuida et al., 1997) including activation of intracellular signaling pathways such as MAP-kinase and/or NF-ï«B (Hofmann et al., 1999; Arumugam et al., 2004; Zenz et al., 2005).
Calprotectin is produced abundantly and constitutively in the neutrophils and monocytes. This pro-inflammatory protein is expressed also in the squamous mucosal keratinocytes and innate immune cells present at the surface of mucosal tissue (Hsu et al., 2009). In the patient suffering from inflammatory diseases, the calprotectin level in the plasma, feces, dental calculus, gingival crevicular fluid, and saliva changes (Kido, et al., 2003). Especially in rheumatoid arthritis, the calprotectin level in the synovial fluid is increased (Hammer, et al. 2011). During inflammation, calprotectin expression is induced in the epidermal keratinocytes, gastrointestinal epithelial cells and fibroblasts (Hsu et al., 2009). Since the expression or induction of calprotectin in the salivary gland is important in terms of the oral defense system, the regulation of its expression was studied previously (Javkhlan et al., 2011). In this previous study, we showed that mRNAs and proteins for both S100A8 and S100A9 are induced by LPS via Toll-like receptor 4 (TLR4) in the mouse submandibular and parotid glands (SMG and PG, respectively). We also noticed that S100A8 and S100A9 were elevated to a small degree by LPS in the same glands in LPS hyposensitive TLR4-mutant C3H/HeJ mice, implying the possibility of presence of LPS-signaling(s) other than the one via TLR4 in the salivary glands (Javkhlan et al., 2011).
The family of nucleotide-binding oligomerization domain-containing protein-like receptors (NLRs) contains modules of the nucleotide-binding oligomerization domain (NOD) and belongs to a group of intracellular pattern-recognition receptors (Inohara et al., 2005). NOD-containing protein (Nod) 1 and Nod2 are the most studied members of the NLR family, and it is well known that mutations in the Nod2 gene are associated with Crohn’s disease (Ogura et al., 2001a; Hugot et al., 2001). In contrast, its up-regulation is associated with Blau’s syndrome (Miceli-Richard et al., 2001). The ligands for Nod1 and Nod2 are bacterial molecules containing the D-glutamyl-meso-diaminopimelic acid moiety and muramyl dipeptide (MDP), respectively. The major responses upon challenges of these ligand via cytosolic Nod1 and Nod2 are activation of NF-ï«B through IKK (Strober et al., 2006). In macrophages, one of these molecules, Nod2, is reported to respond to LPS as well as to MDP (Pauleau and Murra, 2003).
In the present study, therefore, we examined if the expression of salivary gland S100A8 and S100A9 is also regulated by Nod2 or by other members of the NLR family, since these subunits (S100A8 and S100A9) of the inflammatory protein calprotectin, though at low level, respond to LPS in the salivary glands lacking normal TLR4 expression (Javkhlan et al., 2011).
Materials and Methods
Reagents
LPS (Escherichia coli serotype 0111:B4), Tri-ReagentTM, cycloheximide (CHX), mouse monoclonal anti-ï¢-actin-peroxidase, and sets of primers for RT-PCR and quantitative real-time RT-PCR were obtained from Sigma-Aldrich (St. Louis, MO, USA). SuperscriptTM One Step RT-PCR with the Platinum Taq System was obtained from Invitrogen (Carlsbad, CA, USA), whereas SYBR Green PrimescriptTM RT-PCR kit (Perfect Real Time) was purchased from TaKaRa (Shiga, Japan).
Complete EDTA-free protease inhibitor cocktail tablets were obtained from Boehringer Mannheim (Mannheim, Germany). Goat anti-Nod1 antibody came from Santa Cruz (Santa Cruz, CA, USA); and goat anti-Nod2 antibody, from IMGENEX (San Diego, CA, USA). Rabbit anti-goat IgG horseradish peroxidase conjugate and Immobilon-P transfer membranes were purchased from Beckman Coulter (Brea, CA, USA) and Millipore Co. (Billerica, MA, USA), respectively. Can Get Signal was obtained from Toyobo Co. Ltd. (Osaka, Japan), whereas an Enhanced Chemical Luminescence (ECL) Detection Kit was purchased from GE Healthcare (Buckinghamshire, UK).
Animals and LPS/CHX Treatments
Three pairs of TLR4-knockout, C57BL/6 (TLR4-/-) mice were obtained from OrientalBioService, Inc. (Kyoto, Japan); they were mated in our animal facility to obtain off-springs. The wild-type, C57BL/6NCrSn (TLR4+/+) male mice, aged 7-8 weeks were purchased from Japan SLC, Inc. (Shizuoka, Japan). They will be simply designated as TLR4-knockout (TLR4-KO) and wild-type (WT) mice in this paper. All animals were housed under standard conditions (12-h light/12-h darkness cycle at 22-25ï‚°C) with free access to food and water. They were used for experiments at the age of 8 to 9 weeks.
In the RT-PCR experiment to estimate the salivary gland mRNA levels of S100A8, S100A9, IL-1ï¢, Nod1/Nod2, TLR4, and ï¢-actin, mice were injected i.p. with LPS dissolved in saline at a dose of 0.4 mg/kg body weight (Yao et al., 2005a); and control animals, with saline. The animals were euthanized by cervical dislocation at 3 h after the saline- or LPS-treatment. In the quantitative real-time RT-PCR experiment to examine the effects of LPS and CHX on the mRNA levels of S100A8 and S100A9, animals were divided into 4 groups and injected with CHX and LPS either singly or in combination. CHX or control saline was injected into mice i.p., 30 min prior to LPS injection; and the animals were euthanized by cervical dislocation at 3 h after the LPS injection. Dosages of CHX and LPS were 50 mg (Tait et al., 2005) and 0.4 mg/kg body weight, respectively.
In the Western blotting experiment to examine the effects of CHX and LPS on the Nod1 and Nod2 protein levels, animals were divided into 4 groups, each given these 2 reagents singly or in combination similarly as described above.
In all of the above experiments, each group consisted of 4-5 mice for quantitative analyses; whereas 2-3 mice were used for other assays. The protocols used in the present animal experiments were approved by the Institutional Review Board of the Animal Committee of The University of Tokushima.
Preparation of Total RNA, RT-PCR, and Quantitative Real-Time RT-PCR
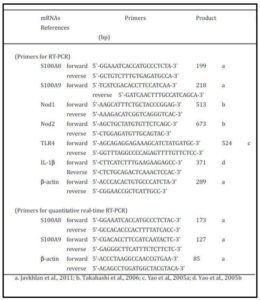
The mice were euthanized by cervical dislocation and the SMG and PG were carefully removed from each mouse. Special care was taken when the SMG were dissected from sublingual gland. The removed SMG and PG were used for isolation of total RNA with Tri-reagent by the standard procedure. RT-PCR amplification of mRNAs for S100A8, S100A9, IL-1ï¢, Nod1, Nod2, and ï¢-actin was performed as described earlier (Javkhlan et al 2011; Yao et al., 2010). Briefly, RT-PCR amplification of S100A8 and S100A9 mRNAs was mostly performed by using the SuperScript™ One Step RT-PCR system in a thermal cycler (TaKaRa Thermal Cycler MP, model TP 3000, Shiga, Japan). The primers used were prepared according to the published sequences (Javkhlan et al 2011; Yao et al., 2005b; Takahashi et al., 2006; Yao et al., 2005a; Table 1).
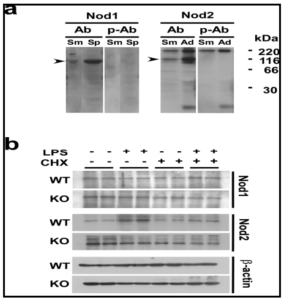
The specificity of antibodies was first confirmed by the Fig. 4a, b experiment shown in Fig. 4a, where Nod1 and Nod2 proteins at approximate molecular masses of 96-kDa and 115-kDa, respectively, were specifically detected. These sizes well agreed with the ones reported previously (Inohara et al., 1999; Ogura et al., 2011b). The effects of CHX on LPS-induced changes in Nod1 and Nod2 protein levels are shown in Fig. 4b. In both WT and TLR4-KO mice, the Nod1 protein level in the SMG did not change in response to the single or combined injection of LPS and CHX. However, the Nod2 protein level in the WT mice was markedly increased by the injection of LPS; and this increase was completely inhibited by the pre-treatment of the animals with CHX (Fig. 4b). On the other hand, in the TLR4-KO mice, the profile of changes in the Nod2 protein level was completely different from that of the WT mice: First, the level was not increased by the LPS injection, either in the presence or absence of CHX. Secondly, the Nod2 protein level was higher in the TLR4-KO control than in the WT one. The results in Fig. 4 are well consistent with the Nod1/Nod2 mRNA data shown in Fig. 2a.
Discussion
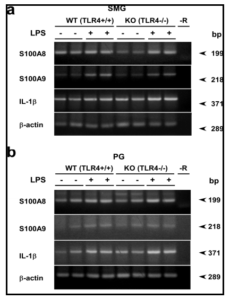
Using LPS-hyporesponsive TLR4-mutant C3H/HeJ and their WT counterpart, it was previously shown that mRNA and protein levels of S100A8 and S100A9, i.e., subunits of calprotectin, in the SMG and PG are increased by treatment with LPS (Javkhlan et al., 2011). This increase is mostly mediated via TLR4, but a small increase was also noticed in S100A8 and S100A9 mRNA levels in response to LPS in the salivary glands of even C3H/HeJ mice. It is well known that C3H/HeJ mice bear a point mutation in their TLR4 gene, leading to the replacement of a proline with a histidine at position 712 in the TLR4 protein molecule (Poltorak et al., 1998). This mutation then causes dysfunction of TLR4. Yet, it cannot be ruled out whether genes other than TLR4 are not also affected in C3H/HeJ mice. In the present study, we employed TLR4-KO and their WT mice in order to avoid unnecessary confusion due to unanticipated gene mutation that might occur in C3H/HeJ mice. As shown by the TLR4 amplification experiment, the lack of TLR4 mRNA in the TLR4-KO mice and its expression in the WT ones (C57BL/6 strain) were confirmed (Fig. 2); thus the differences observed between WT and TLR4-KO mice were obviously mediated via TLR4. In the SMG and PG of TLR4-KO and control WT mice, LPS consistently increased the mRNA and protein levels of S100A8 and S100A9, supporting the previous data (Javkhlan et al., 2011).
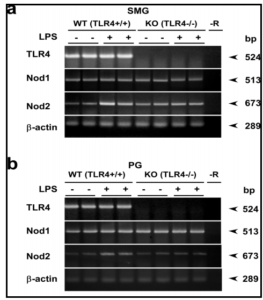
Here, we focused on the NLRs, particularly, Nod1 and Nod2, in the salivary gland in relation to the S100A8/A9 induction, since one of them, Nod2, in macrophages is reported to respond to LPS and to activate NF-ï«B similarly as signaling via TLR4 (Pauleau and Murra, 2003). In the present study, we found for the first time that 1) Nod2 mRNA and protein were expressed in the SMG, one of the major salivary glands. 2) LPS strongly induced the Nod2 mRNA and protein in WT but not in TLR4-KO mice, indicating that this endotoxin activated Nod2 transcription via the TLR4 signaling in the salivary gland. CHX actually inhibited LPS-induced elevation of the Nod2 protein in the SMG in WT mice. Also, 3) levels of both mRNA and protein of Nod2 in this tissue were significantly higher in the TLR4-KO mice than those in WT mice.
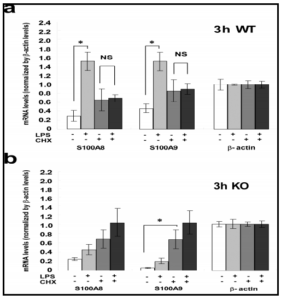
In the SMG of WT mice, marked elevations of mRNA levels for S100A8 and S100A9 elicited by LPS were significantly blocked by the presence of CHX; these changes and changes in the levels of Nod2 mRNA/protein were in parallel. These results suggest that Nod2, increased in expression by LPS, may have activated the transcription of mRNAs of S100A8 and S100A9. The fact implies a possible involvement of Nod2 protein in the induction of S100A8/S100A9 mRNAs by LPS. Although this implication is still preliminary and needs more experiments using a specific inhibitor of Nod2 or using Nod2-KO animals, the present study tentatively proposes the hypothesis regarding the relation between Nod2 and S100A8/A9 in response to LPS stimulation.
In the SMG of TLR4-KO mice a higher level of Nod2 was expressed than that observed in WT mice. This fact is well supported by the finding that macrophages or mice made insensitive to TLRs potentially respond to Nod2 stimulation (Kim et al., 2008). Also, the Nod2 protein level was not altered by CXH treatment in the SMG of TLR4-KO mice, implying the constitutively high expression of Nod2 in these mice. Therefore, regardless of the presence or absence of CHX, the low level-elevation of the mRNAs for S100A8 and S100A9 caused by LPS may be elucidated to have occurred via Nod2. The hypothesis that LPS directly activates Nod2 needs to be verified using TLR4-KO mice.
Another issue that has arisen in the present study is the effects of CHX on the S100A8 and S100A9 mRNA levels in the SMG. In both TLR4-KO and WT mice, CHX itself elevated the mRNA levels for S100A8 and S100A9. The reason for this increase is unclear at present; but we hypothesize the presence of some constitutively expressed putative suppressor of S100A8 and S100A9 transcription with short half life (as the effect was seen in 3 h), the synthesis of which was inhibited by CHX. This way, the elevation by CHX of S100A8/A9 mRNAs in the SMG in WT and TLR4-KO mice (Fig. 3) could be well elucidated.
Lastly, in TLR4-KO mice, a higher level of Nod2 is expressed, and though at small degree, the S100A8 and S100A9 mRNA are induced by LPS. Nod2 seems to play a pivotal role in the LPS signaling in the salivary gland as well in the case where the TLR4 pathway is insensitive. Thus, calprotectin hetero subunits, S100A8 and S100A9, in the SMG seemed to have increased in expression by LPS via the Nod2 signaling pathway in addition to the TLR4 one. In WT mice, having both of these signaling pathways, the S100A8 and S100A9 mRNA inductions are potentiated in this tissue.
We conducted the present experiment by using CHX to block Nod2 protein synthesis. In our present study, CHX successfully inhibited Nod2 protein elevation in the WT mice, though the inhibitor would not be specific to Nod2 protein synthesis. Before drawing final conclusions, it would be important to confirm our present data by performing experiments using Nod2-specific inhibitors (Huang et al., 2008; Bielig et al., 2010) and such inhibitors are currently under investigation in our laboratory.
Acknowledgement
The authors are grateful to Dr. Makoto Fukui, Department of Preventive Dentistry, Institute of Health Biosciences, The University of Tokushima Graduate School, for his help in the statistical analysis.
References
1. Arumugam, T., Simeone, D.M., Schmidt, A.M. and Logsdon, C.D. (2004) “S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE),” J Biol Chem, 279: 5059-5065.
Publisher – Google Scholar
2. Bielig, H., Velder, J., Saiai, A., Menning, M., Meemboor, S., Kalka-Moll, W., Krcnke, M., Schmalz, H.-G. and Kufer, T. A., (2010) “Anti-inflammatory arene-chromium complexes acting as specific inhibitors of NOD2 signalling,” Chem Med Chem, 5: 2065-2071.
Publisher – Google Scholar
3. Bradford, M.M. (1976) “Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,” Anal Biochem, 72: 248-254.
Google Scholar
4. Cuida, M., Halse, A.K., Johannessen, A.C., Tynning, T. and Jonsson, R. (1997) “Indicators of salivary gland inflammation in primary Sjögren’s syndrome,” Eur J Oral Sci, 105: 228-233.
Publisher – Google Scholar
5. Donato, R. (2001) “S100 : a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles,” Int J Biochem Cell Biol, 33: 637-668.
Publisher – Google Scholar
6. Hammer, H.B., Fagerhol, M.K., Wien, T.N., and Kvien, T.K. (2011) The soluble biomarker calprotectin (a S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab Arth Res Ther, 13: R178.
7. Hofmann, M.A., Drury, S., Fu, C., Qu, W., Taguchi, A., Lu., Avila, C., Kambham, N., Bierhaus, A., Nawroth, P., Neurath, M.F., Slattery, T., Beach, D., McClary, J., Nagashima, M., Morser, J., Stern, D. and Schmidt, A.M. (1999) “RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides,” Cell, 97: 889-901.
Publisher – Google Scholar
8. Hsu, K., Champaiboon, C., Guenther, B.D., Sorenson, B.S., Khammanivong, A., Ross, K.F., Geczy, C.L. and Herzberg, M.C. (2009) “Anti-infective protective properties of S100 calgranulins,” Antiinflamm Antiallergy Agents Med Chem, 8: 290-305.
Publisher – Google Scholar
9. Huang, S., Zhao, L., Kim, K., Lee, D.S. and Hwang, D.H. (2008) “Inhibition of Nod2 signaling and target gene expression by curcumin,” Mol Pharmacol, 74: 274-281.
Publisher – Google Scholar
10. Hugot, J.P., Chamaillard, M., Zouali, H., Lesage, S., Cézard, J.P., Belaiche, J., Almer, S., Tysk, C., O’Morain, C.A., Gassull, M., Binder, V., Finkel, Y., Cortot, A., Modigliani, R., Laurent-Puig, P., Gower-Rousseau, C., Macry, J., Colombel, J.F., Sahbatou, M. and Thomas, G. (2001) “Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease,” Nature, 411: 599-603.
Publisher – Google Scholar
11. Inohara, N., Chamailland, M., MacDonald, C. and Núñets, G. (2005) “NOD-LRR proteins: role in host-microbial interactions and inflammatory disease,” Annu Rev Biochem, 74: 355-383.
Publisher – Google Scholar
12. Inohara, N., Koseki, T., del Peso, L., Hu, Y., Yee, C., Chen, S., Carrio, R., Merino, J., Liu, D., Ni, J. and Núñez, G. (1999) “Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-ï«B,” J Biol Chem, 274: 14560-1457.
Publisher – Google Scholar
13. Inohara, N., Ogura, Y., Chen, F.F., Muto, A. and Nuñez, G. (2001) “Human Nod1 confers responsiveness to bacterial lipopolysaccharides,” J Biol Chem, 276: 2551-2554.
Google Scholar
14. Javkhlan, P., Hiroshima, Y., Azlina, A., Hasegawa, T., Yao, C., Akamatsu, T., Kido, J., Nagata, T. and Hosoi, K. (2011) “Lipopolysaccharide-mediated induction of calprotectin in the submandibular and parotid glands of mice,” Inflammation, 34: 668-680.
Publisher – Google Scholar
15. Kido, J., Kido, R. Suryono, Kataoka, M., Fagerhol, M.K., and Nagata. T., 2003. Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-Tolllikereceptor-nuclear factor κB pathway. J. Perio. Res. 38: 557-563.
Publisher – Google Scholar
16. Kim, Y.-G., Park, J.-H., Shaw, M.H., Franchi, L., Inohara, N. and Nuñez, G. (2008) “The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands,” Immunity, 28: 246-257.
Publisher – Google Scholar
17. Miceli-Richard, C., Lesage, S., Rybojad, M., Prieur, A.M., Manouvrier-Hanu, S., Häfner, R., Chamaillard, M., Zouali, H., Thomas, G. and Hugot, J.P. (2001) “CARD15 mutations in Blau syndrome,” Nature Genet, 29: 19-20.
Publisher – Google Scholar
18. Ogura, Y., Bonen, D.K., Inohara, N., Nicolae, D.L., Chen, F.F., Ramos, R., Britton, H., Moran, T., Karaliuskas, R., Duerr, R.H., Achkar, J.P., Brant, S.R., Bayless, T.M., Kirschner, B.S., Hanauer, S.B., Nuñez, G. and Cho, J.H. (2001a) “A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease,” Nature, 411: 603-606.
Publisher – Google Scholar
19. Ogura, Y., Inohara, N., Benito, A., Chen, F.F., Yamaoka, S. and Núñez, G. (2001b) “Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-ï«B,” J Biol Chem, 276: 4812-4818.
Publisher – Google Scholar
20. Pauleau, A.-L. and Murra, P.J. (2003) “Role of Nod2 in the response of macrophages to Toll-like receptor agonists,” Mol Cell Biol, 23: 7531-7539.
Publisher – Google Scholar
21. Poltorak, A., He, X. and Smirnova, I. (1998) “Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene,” Science 282: 2085-2088.
Publisher – Google Scholar
22. Pouliot, P., Plante, I., Raquil, M.-A., Tessier, P. and Olivier, M. (2008) “Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response,” J Immunol, 181: 3595-3601.
Google Scholar
23. Ryckman, C., Vandal, K., Roulean, P., Talbot, M. and Tessier, P.A. (2003) “Proinflammatory activities of S100: Proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion,” J Immunol, 170: 3233-3242.
Publisher – Google Scholar
24. Stříž, I. and Trebichavský, I. (2004) “Calprotectin – a pleiotropic molecule in acute and chronic inflammation,” Physiol Res, 53: 245-253.
Google Scholar
25. Strober, W., Murray, P.J., Atsushi Kitani, A., and Watanabe, T. (2006) “Signalling pathways and molecular interactions of NOD1 and NOD2,” Nature Reviews Immunology, 6: 9-20.
Publisher – Google Scholar
26. Takahashi, Y., Isuzugawa, K., Murase, Y., Imai, M., Yamamoto, S., Iizuka, M., Akira, S., Bahr, G.M., Momotani, E., Hori, M., Ozaki, H. and Imakawa, K. (2006) “Up-regulation of NOD1 and NOD2 through TLR4 and TNF-alpha in LPS-treated murine macrophages,” J Vet Med Sci, 68: 471-478.
Publisher – Google Scholar
27. Tait J.F., Smith C., and Blankenberg F.G. (2005) “Structural requirements for in vivo detection of cell death with 99mTc-annexin V,” J Nucl Med, 46: 807-815.
Google Scholar
28. Yao, C., Li, X., Kwartarini, M., Kosugi-Tanaka, C., Akamatsu, T., Kanamori, N. and Hosoi, K. (2005a) “Lipopolysaccharide-induced elevation and secretion of interleukin-1ï¢ in the submandibular gland of male mice,” Immunology, 116: 213-22.
Publisher – Google Scholar
29. Yao, C., Nunuk, P., Karabasil, M.R., Azlina, A., Javkhlan, P., Hasegawa, T., Akamatsu, T., Hosoi, T., Ozawa, K. and Hosoi, K. (2010) “Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-ï«B and p-c-Jun/c-Fos,” Am J Pathol, 177: 724-734.
Publisher – Google Scholar
30. Yao, C., Wei, W., Li, X. and Hosoi, K. (2005b) “Acute phase protein induction by experimental inflammation in the salivary gland,” J Oral Pathol Med, 34: 364-367.
Publisher – Google Scholar
31. Zenz, R., Eferl, R., Kenner, L., Florin, L., Hummerich, L., Mehic, D., Scheuch, H., Zenz, R., Eferl, R., Kenner, L., Florin, L., Hummerich, L., Mehic, D. and Scheuch, H. (2005) “Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins,” Nature, 437: 369-375.
Publisher – Google Scholar