Introduction
Diabetes is devastating disease related to glucose intolerance either due to decreased production of insulin or increased resistance to peripheral insulin receptors (Babenko et al., 2006; Wild et al., 2004). Recently, the new types of this diseases have been revealed as mutation of SUR or Kir subunits of ATP-sensitive K+ channels (KATP ) leading to malfunction of release mechanism for insulin from pancreas while number and function of beta-cells and insulin receptors are unchanged (Gloyn et al., 2004). Usually, chronicle diabetes which is not treated properly leads to microangiopathy, neuropathy and the number of pathological changes as the consequences of toxic effects of glycosilated proteins. Hypertension and coronary heart disease are frequently associated with diabetes, however it is not clear what is the precise mechanism of the genesis of these diseases. Possible involvement of KATP channels as one of the basic mechanism for regulation contractility of the vascular smooth muscles should be taken into account. Furthermore, frequently reported gender differences in this aspect remain to be elucidated (Ranki et al.,, 2001). KATP channels openers are useful experimental tools for evaluation of functional activity of these channels, however many of them are not selective and have some additional mechanism of action (Adebiyi et al., 2008). Activation of KATP channels in vascular smooth muscle induced endothelium-independent vasodilation which is glibenclamide-sensitive. According to our previous data, diazoxide at concentration 100 μM induces glibenclamide-sensitive vasodilation in guinea pig aorta which is gender and season-independent, opposite to pinacidil (Kocic and Gruchala-Niedoszytko, 2009). Therfore, in the present study we used phenylephrine to induce contraction of isolated rat tail artery and diazoxide to relax them in healthy rats and rats with strptozotocin-induced diabetes of both genders.
Material and Methods
All experiments were performed on Wistar rats of both genders, weighing between 200 and 300 g, kept under standard laboratory conditions. All animals received humane care in accordance with recommendation of local Ethic Committee.
Experimental Design
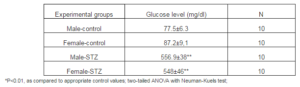
All animals were divided in four groups: males, control (MC) and experimental group with streptazotocin induced diabetes (MD), and females, control (FC) and with a diabetes (FD). Diabetes was induced by one i.p. injection of streptozotocin (75 mg/kg B.W.), according to well established experimental model (Rakieten et al., 1963). After 10 days blood samples were taken and glucose levels determined (see Table 1). After 3 weeks, animals were sacrificed after anesthesia by pentobarbital (60 mg/kg i.p.) and the segments of the ventral portion of the tail artery were removed carefully, cleaned of adhering tissue and cut into 3 to 5 mm rings, then mounted under optimal tension in 10ml bath containing solution of the following composition: 119 mM NaCl, 4.7 mM KCl, 2.5mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 11 mM glucose. Solution was kept at 37ï‚° and aerated by carbogen (5% CO2 and 95% O2) and attached to the pressure transducer (P23 Db, Statham Laboratories, USA), with a preload 0.5g. (Kocic et al., 2010). Peristaltic pump (PP1-05, Poland) supported perfusion of the fragment of artery with a velocity from 0.4 ml/min in the beginning of the experiments up to the 1,6ml/min during application of the drugs. After incubation taken about 90 to 120 min, all preparations were treated by 1) phenylephrine (raising concentrations from 0.1 to 300 µM) alone; 2) phenylephrine in the presence of diazoxide at 100 µM. This procedure is repeated in diabetic groups of the male and female rats.
Table1. The Levels of Glucose In Male and Female Rats before (Control) and 10 Days after Treatment with Streptozotocin (STZ) at Dose 75mg/kg B.W. i.v.
Drugs
All drugs (phenylephrine and diazoxide) were purchased from SIGMA and dissolved in distilled water.
Statistics
Results were expressed as mean  SE. Concentration-response curves and maximal effects were analyzed by software Pharma/PCS version4 (Pharmacological Calculations System). Statistical significance of the differences between means were determined by student t-test for paired data, or by ANOVA with multiple comparison Neuman-Keuls test. P<0.05 was taken as the level of significance.
Results and Discussion
In this paper we present gender differences in vasodilation effects of ATP-sensitive potassium channel opener diazoxide in diabetic male and female rats using isolated tail arteries as experimental model. Based on facts, that we applied diazoxide, which is known as a strong activator of ATP-sensitive K+ channels (KATP) in smooth muscle, confirmed in our previous research (Kocic et al, 2006; Kocic and Gruchala-Niedoszytko 2009), and results obtained in control groups with phenylephrine in the presence of mentioned activator of KATP, we set up the hypothesis that SID downregulates expression of KATP or induced some disturbances in signaling pathway related to KATP channels, especially in males rats. Obviously, the main limitation of this statement is that we have not determined expression of the subunits of KATP yet. However, our simple study indicate two possibilities for measured significantly stronger maximal effects of phenylephrine in males with SID then in males control in the presence of diazoxide. First, that it is related to overexpression of α1 adrenergic receptors, and, second, that it is due to downregulation of KATP or their resistance to diazoxide in the presence of significant hyperglycemia. Diazoxide failed to diminish maximal contraction effects induced by phenylephrine only in male rats with STZ-induced diabetes. On the contrary, in females, contribution of KATP in regulation of smooth muscle tonus seems to be unchanged in control and SID animals (Fig 2). Although, confirmation of this observation by blotting analysis of expression of SUR and Kir subunits in the tail arteries smooth muscles is mandatory. Noteworthy, there was already evidenced that SUR subunits of ATP-sensitive potassium channels can be downregulated in a gender-dependent way (Ranki et al., 2001).
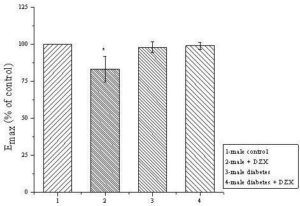
Fig. 1. The Maximal Effects of Phenylephrine: 1: in Male Control Group Taking as 100%; 2: in Control Group in the Presence of 100 μM of Diazoxide; 3: in SID Group; 4: in SID Group in the Presence of Diazoxide (DZX) on the Amplitude of Contraction of Tail Arteries Obtained from Different Groups of Rats.
*P<0, 05: statistical significance as compared to control male group. N=10;
SID-streptozotocin induced diabetes
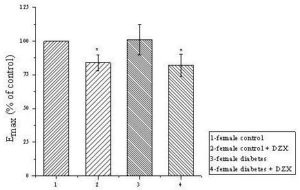
Fig. 2. The Maximal Effects of Phenylephrine in 1: Female Control Group Taken as 100%; 2: in Control Group in the Presence of 100 μM of Diazoxide; 3: in SID Group; 4: in SID Group in the Presence of Diazoxide (DZX) on the Amplitude of Contraction of Tail Arteries Obtained from Different Groups of Rats.
*P<0, 05: statistical significance as compared to control female group. N=10;
SID-streptozotocin induced diabetes
Nevertheless, there are several critical points of our pharmacological study requiring analysis. Firstly, the mechanism of diazoxide-induced vasodilation (or diminishing of phenylephrine-induced contraction of rat tail artery) should be discussed. We assumed that vasodilation is due to activation of KATP channels based on our previous study demonstrating glibenclamide-sensitive vasodilation of aorta in male and female guinea pigs (Kocic and Gruchala-Niedoszytko, 2009). Additionally, there is an evidence that stimulation of α1 adrenergic receptors and activation of 3-kinase-Akt pathway can modulate function of vascular KATP channels in rat thoracic aorta (Haba et al., 2010), but it was related to vasodilation induced by levcromakalim. Moreover, it cannot explain gender difference observed in our study only in the presence of diazoxide in diabetic male rats.
To conclude, our study indicate that SID in rats leads to significant lower responsiveness of vascular smooth muscles to diazoxide in males only, which suggests diminishing participation of KATP channels in regulation of vascular smooth muscle relaxation due to highly significant hyperglycemia. The reason for gender differences in this phenomenon remains to be elucidated.
References
Adebiyi, A., McNally, E. M. & Jaggar, J. H. (2008). “Sulfonylurea Receptor-Dependent and —Independent Pathways Mediate Vasodilation Induced by ATP-Sensitive K+ Channels Openers,” Molecular Pharmacology, 74 (3) 736-743.
Publisher – Google Scholar
Babenko, A. P., Polak, M., Cave, H., Busiah, K., Czernichow, P., Scharfmann, R., Bryan, J., Aguilar-Bryan, L., Vaxillaire, M. & Froguel, P. (2006). “Activating Mutations in the ABCC8 Gene in Neonatal Diabetes Mellitus,” New England Journal of Medicine, 355 (5) 456-466.
Publisher – Google Scholar
Gloyn, A. L., Pearson, E. R., Antcliff, J. F., Proks, P., Bruining, G. J., Slingerland, A. S., Howard, N., Srinivasan, S., Silva, J. M., Molnes, J., Edghill, E. L., Frayling, T. M., TempleI, K., Mackay, D., Shield, J. P., Sumnik, Z., van Rhijn, A., Wales, J. K., Clark, P., Gorman, S., Aisenberg, J., Ellard, S., Njølstad, P. R., Ashcroft, F. M. & Hattersley, A. T. (2004). “Activating Mutations in the Gene Encoding the ATP-Sensitiv Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes,” New England Journal of Medicine, 350 (14) 1838-1849.
Publisher – Google Scholar
Haba, M., Hatakeyama, N., Kinoshita, H., Teramae, H., Azma, T., Hatano, Y. & Matsuda, N. (2010). “The Modulation of Vascular ATP-Sensitive K+ Channel Function via the Phosphatidylinositol 3-Kinase-Akt Pathway Activated by Phenylephrine,” The Journal of Pharmacology and Experumental Therapeutics, 334 673-678.
Publisher – Google Scholar
Kocić, I., Gruchala, M. & Petrusewicz, J. (2006). “Selective Inhibition of Pinacidil Effects by Estrogen in Guinea Pig Heart,” International Journal of Cardiology, 110 (1) 22-26.
Publisher – Google Scholar
Kocić, I. & Gruchała-Niedoszytko, M. (2009). “Pinacidil Relaxing Effect on Phenylephrine-Precontracted Guinea Pig Aorta was Abolished by Pretreatment with 17-Beta-Estradiol,” International Journal of Cardiology, 133 (1) 116-1188.
Publisher – Google Scholar
Kocić, I., Szczepańska, R. & Wapniarska, I. (2010). “Estrogen-Induced Relaxation of the Rat Tail Artery is Attenuated in Rats with Pulmonary Hypertension,” Pharmacological Reports, 62 (1) 95-99.
Publisher – Google Scholar
Rakieten, N., Rakieten, M. L. & Nadkarni M. R. (1963). “Studies on the Diabetogenic Action of Streptozotocin,” Cancer and Chemotherapy Reports, 29 91-98.
Publisher – Google Scholar
Ranki, H. J., Budas, G. R., Crawford, R. M. & Jovanović, A. (2001). “Gender-Specific Difference in Cardiac ATP-Sensitive K(+) Channels,” Journal of American College of Cardiology, 38 (3) 906-915.
Publisher – Google Scholar
Wild, S., Roglic, G., Green, A., Sicree, R. & King, H. (2004). “Global Prevalence of Diabetes: Estimates for the Year 2000 and Projection for 2030,” Diabetes Care, 27 (5) 1047-1053.
Publisher – Google Scholar





