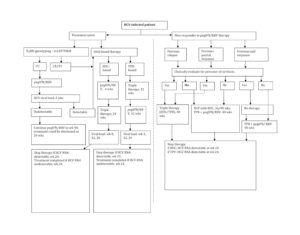
Additional Cost Burden on the Use of Newly Emerged DAA for Therapy
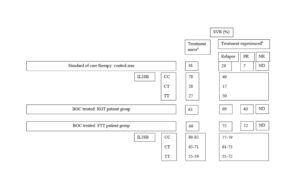
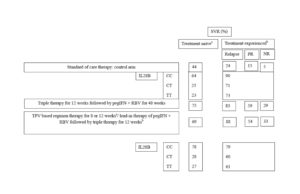
Although data from clinical trials on the use of DAA for patient management have been encouraging, its use in routine clinical practice would be highly dependent on the additional cost involved without compromising on the SVR rate. An economic analysis assessing the average cost estimates involving PI-based regimens (and compensating for treatment of adverse events suffered) revealed that PI-based therapies were cost-effective in comparison to the recommended SOC therapy (Camma, 2012). The total average cost of BOC-RGT in comparison to standard duration with BOC was found to be £22,850 vs £34,680 and £25,060 vs £34,350 for treatment-naïve and treatment-experienced patients, respectively. Similarly, the total average cost of response-guided therapy with telaprevir vs standard duration telaprevir was found to be £29,930 vs £32,530, and £31,880 vs £31,680 for treatment-naïve and treatment-experienced patients, respectively (Camma, 2012).
Duration of DAA-Based Treatment Regimen
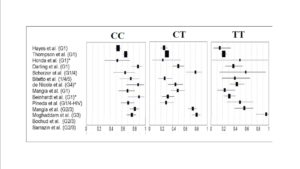
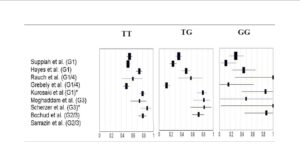
Data from DAA-based clinical trials have revealed that >90% of patients with favorable CC-genotype (rs12979860) effectively achieved SVR and were eligible for a 24-week therapy (Jacobson, 2011b); while patients with non-CC genotypes and especially non-responders were reported to have lower SVR rates similar to those being treated with SOC therapy (Jacobson, 2011a). Hence, there seems to be a need for designing better effective treatment regimens or design dual/triple/quadruple-DAA based regimens to achieve higher SVR rates, which in turn would be associated to severe adverse events and increased risk of resistance to PI.
Lead-in SOC Therapy
The clinical relevance of lead-in therapy of SOC regimen would be more significant in patients with non-CC genotype (rs12979860); wherein patients achieving SVR would continue on non-DAA based regimen; while those not achieving SVR could be initiated on an expensive treatment regimen with DAA.
Conclusion
The changing landscape for anti-HCV therapy was marked by the introduction of pharmacogenomic-based predictive therapy (IL28B genotyping) and clinical use of DAAs (boceprevir and telaprevir). Reports from recent clinical trials have proven synergic use of IL28B genotypic results with DAA-based therapy to design personalized treatment regimens and achieve higher SVR rates. Also, a large proportion of previously treated pegIFN/RBV non-responders and relapsers can now be effectively treated with DAA-based treatment regimens thereby increasing SVR rates. But with ongoing evaluation of second-generation DAAs, the future of pharmacogenomic-based therapy in clinical practice remains uncertain.
References
1. Akuta, N., Suzuki, F., Hirakawa, M., Kawamura, Y., Yatsuji, H., Sezaki, H., Suzuki, Y., Hosaka, T., Kobayashi, M., Saitoh, S., Arase, Y., Ikeda, K., Kumada, H. (2010). “Amino acid substitutions in the hepatitis C virus core region of genotype 1b affect very early viral dynamics during treatment with telaprevir, peginterferon, and ribavirin,” Journal of Medical Virology. 82, Pp. 575-582.
Publisher – Google Scholar
2. Akuta, N., Suzuki, F., Sezaki, H., Suzuki, Y., Hosaka, T., Someya, T., Kobayashi, M., Saitoh, S., Watahiki, S., Sato, J., Matsuda, M., Kobayashi, M., Arase, Y., Ikeda, K., Kumada, H. (2005). “Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon-ribavirin combination therapy,” Intervirology, 48, Pp. 372-380.
Publisher – Google Scholar
3. Alestig, E., Arnholm, B., Eilard, A., Lagging, M., Nilsson, S., Norkrans, G., Wahlberg, T., Wejstål, R., Westin, J. & Lindh, M. (2011). “Core mutations, IL28B polymorphisms and response to peginterferon/ribavirin treatment in Swedish patients with hepatitis C virus genotype 1 infection,” BMC Infectious Diseases, 11, Pp. 124-130.
Publisher – Google Scholar
4. Antaki, N., Bibert, S., Kebbewar, K., Asaad, F., Baroudi, O., Alideeb, S., Hadad,. M,., Abboud D, Sabah, H., Bochud,.P. Y., Negro F. (2012). “IL28B polymorphisms do not predict response to therapy in chronic hepatitis C with HCV genotype 5,” Gut, 61, Pp. 1640—1641.
Publisher – Google Scholar
5. Asselah, T., Muvnck, S. D., Broet, P., Planchon, J. M., Blanluet, M., Bieche, I., Lapalus, M., Peignoux, M. M., Lada, O., Estrabaud, E., Zhang, Q., Ray, A. E., Vidaud, D., Ripault, M. P., Boyer, N., Bedossa, P., Valla, D., Vidaud, M. & Marcellin P. (2012). “IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C,” Journal of Hepatology, 56(3), Pp. 527-532.
Publisher – Google Scholar
6. Bacon, B. R., Gordon, S. C., Lawitz, E., Marcellin, P., Vierling, J. M., Zeuzem, S., Poordad, F., Goodman, Z. D., Sings, H. L., Boparai, N., Burroughs, M., Brass, C. A., Albrecht, J. K., & Esteban R. (2011). “Boceprevir for previously treated chronic HCV genotype 1 infection,” New England Journal of Medicine, 364, Pp. 1207—1217.
Publisher – Google Scholar
7. Beinhardt, S., Aberle, J. H., Strasser, M., Dulic—Lakovic, E., Maieron, A., Kreil, A., Rutter, K., Staettermayer A. F., Datz, C., Scherzer, T. M., Strassl, R., Bischof, M., Stauber, R., Bodlaj, G., Laferl, H., Holzmann, H., Munda, P. S., Ferenco, P. & Hofer, H. (2012). “Serum Level of IP-10 Increases Predictive Value of IL28B Polymorphisms for Spontaneous Clearance of Acute HCV Infection,” Gastroenterology, 142(1), Pp. 78-85.
Publisher – Google Scholar
8. Bota, S., Sporea, I., Sirli, R., Neghina, A. M., Popescu, A. & Strain, M. (2013). “Role of Interleukin-28B Polymorphism as a Predictor of Sustained Virological Response in Patients with Chronic Hepatitis C Treated with Triple Therapy: A Systematic Review and Meta-Analysis,” Clinical Drug Investigation, 33(5), Pp. 325-331.
Publisher – Google Scholar
9. Butt, A. S., Mumtaz, K., Aqeel, I., Shah, H. A., Hamid, S. & Jafri, W. (2009). “Sustained virological response to pegylated interferon and ribavirin in patients with genotype 3 HCV cirrhosis,” Tropical Gastroenterology, 30(4), Pp. 207—212.
10. Camma, C., Petta, S., Enea, M., Bruno, R., Bronte, F., Capursi, V., Cicchetti, A., Colombo, G. L., Marco, V. D., Gasbarrini, A. & Craxı, A. (2012). “Cost-Effectiveness of Boceprevir or Telaprevir for Untreated Patients With Genotype 1 Chronic Hepatitis C,” Hepatology, 56(3), Pp. 850-860.
Publisher – Google Scholar
11. Cesari, M., Caramma, I., Antinori, S., Adorni, F., Galli, M. & Milazzo, L. (2009). “Impact of hyperglycaemia and cholesterol levels on the outcome of hepatitis C virus (HCV) treatment in HIV/HCV-coinfected patients,” HIV Medicine, 10, Pp. 580—585.
Publisher – Google Scholar
12. Clark, P. J., Thompson, A. J., Zhu, M., Vock, D.M., Zhu, Q., Ge, D., Patel, K., Harrison, S. A., Urban, T. J., Naggie, S., Fellay, J., Tillmann, H. L., Shianna, K., Noviello, S., Pedicone, L. D., Esteban, R., Kwo, P., Sulkowski, M. S., Afdhal, N., Albrecht, J. K., Goldstein, D. B., McHutchison, J. G. & Muir, A. J. (2012). “Interleukin 28B polymorphisms are the only common genetic variants associated with low-density lipoprotein cholesterol (LDL-C) in genotype-1 chronic hepatitis C and determine the association between LDL-C and treatment response,” Journal of Viral Hepatitis, 19(5), Pp. 332-340.
Publisher – Google Scholar
13. Coto-Llerena, M., Perez-Del-Pulgar, S., Crespo, G., Carrión, J. A., Martínez, S. M., Sánchez-Tapias, J. M., Navasa, N. M., & Forns, X. (2011). “Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation,” American Journal of Transplantation, 11(5), Pp. 1051—1057.
Publisher – Google Scholar
14. Crespo, G., Mariño, Z., Navasa, M. & Forns, X. (2012). “Viral Hepatitis in Liver Transplantation,” Gastroenterology, 142, Pp. 1373—1383.
Publisher – Google Scholar
15. Domingo, P., Guardiola, J. M., Salazar, J., Torres, F., Mateo, M. G., Pacho, C., Gutierrez, M. D. M, Lamarca, K., Fontanet, A., Martin, J., Muñoz, J., Vidal, F. & Baigete, M. (2012). “Association of ITPA Gene Polymorphisms and the Risk of Ribavirin-Induced Anemia in HIV/Hepatitis C Virus (HCV)-Coinfected Patients Receiving HCV Combination Therapy,” Antimicrobial Agents and Chemotherapy, 56(6), Pp. 2987-2993.
Publisher – Google Scholar
16. Duarte-Rojo, A., Deneke, M. G. & Charlton, M. R. (2012). “IL28B polymorphism in hepatitis C and liver transplantation,” Liver Transplantation. 19(1), Pp. 49-58.
17. Eskesen, A. N., Melum, E., Moghaddam, A., Bjøro, K., Verbaan, H., Ring-Larsen, H. & Dalgard, O. (2012). “Genetic variants at the ITPA locus protect against ribavirin-induced hemolytic anemia and dose reduction in an HCV G2/G3 cohort,” European Journal of Gastroenterology and Hepatology, 24(8), Pp.890-896.
Publisher – Google Scholar
18. Fellay, J., Thompson, A. J., Ge, D., Gumbs, C. E., Urban, T. J., Shianna, K. V., Little, L. D., Qiu, P., Bertelsen, A. H., Watson, M., Warner, A., Muir, A. J., Brass, C., Albrecht, J., Sulkowski, M., McHutchison, J. G. & Goldstein, D. B. (2010). “ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C,” Nature, 464, Pp. 405-408.
19. Fukuhara, T., Taketomi, A., Motomura, T., Okano, S., Ninomiya, A., Abe, T., Uchiyama, H., Soejima, Y., Shirabe, K., Matsuura, Y., & Maehara, Y. (2010). “Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C,” Gastroenterology, 139(5), Pp. 1577—1585.
Publisher – Google Scholar
20. Ge, D., Fellay, J., Thompson, A. J., Simon, J. S., Shianna, K. V., Urban, T. J., Heinzen, E. L., Qiu, P., Bertelsen, A. H., Muir, A. J., Sulkowski, M., McHutchison, J. G. & Goldstein, D. B. (2009). “Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance,” Nature, 461, Pp. 399-401.
Publisher – Google Scholar
21. Hayashi, K., Katano, Y., Ishigami, M., Itoh, A., Hirooka, Y., Nakano, I., Urano, F., Yoshioka, K., Toyoda, H., Kumada, T. & Goto, H. (2011). “Mutations in the core and NS5A region of hepatitis C virus genotype 1b and correlation with response to pegylated-interferon-alpha 2b and ribavirin combination therapy,” Journal of Viral Hepatitis, 18(4), Pp. 280-286.
Publisher – Google Scholar
22. Hitomi, Y., Cirulli, E. T., Fellay, J., McHutchison, J. G., Thompson, A. J., Gumbs, C. E., Shianna, K. V., Urban, T. J. & Goldstein, D. B. (2011). “Inosine triphosphate protects against ribavirin-induced adenosine triphosphate loss by adenylosuccinate synthase function,” Gastroenterology, 140(4), 1314-1321.
Publisher – Google Scholar
23. Jacobson, I. M., Catlett, I., Marcellin, P. (2011a) “Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial,” Conference Proceedings of 46th Annual Meeting of the European Association for the Study of Liver Diseases (EASL 2011), Abstract 7, Berlin, 30 March — 3 April 2011.
24. Jacobson, I. M., McHutchison, J. G., Dusheiko, G., Bisceglie, A. M. D., Reddy, K. R., Bzowej, N. H., Marcellin, P., Muir, A. J., Ferenci, P., Flisiak, R., George, J., Rizzetto, M., Shouval, D., Sola, R., Terg, R. A., Yoshida, E. M., Adda, N., Bengtsson, L., Sankoh, A. J., Kieffer, T. L., George, S., Kauffman, R. S., & Zeuzem, S. (2011b). “Telaprevir for Previously Untreated Chronic Hepatitis C Virus Infection,” New England Journal of Medicine, 364, Pp. 2405—2416.
Publisher – Google Scholar
25. Jia, Z., Ding, Y., Tian, S., Niu, J. & Jiang, J. (2012). “Test of IL28B Polymorphisms in Chronic Hepatitis C Patients Treated with PegIFN and Ribavirin Depends on HCV Genotypes: Results from a Meta-Analysis,” PLoS ONE, 7(9), e45698.
Publisher – Google Scholar
26. Kurbanov, F., Tanaka, Y., Matsuura, K., Sugauchi, F., Elkady, A., Khan, A., Hasegawa, I., Ohno, T., Tokuda, H., Mizokami, M. (2010). “Positive selection of core 70Q variant genotype 1b hepatitis C virus strains induced by pegylated interferon and ribavirin,” Journal of Infectious Diseases. 201, Pp. 1663-1671.
Publisher – Google Scholar
27. Lagging, M., Askarieh, G., Negro, F., Bibert, S., Soderholm, J., Westin, J., Lindh, M., Romero, A., Missale, G., Ferrari, C., Neumann, A. U., Pawlotsky, J. M., Haagmans, B. L., Zeuzem, S., Bochud, P. Y. & Hellstrand, K. (2011). “Response Prediction in Chronic Hepatitis C by Assessment of IP-10 and IL28B-Related Single Nucleotide Polymorphisms,” PLoS ONE, 6(2), e17232.
Publisher – Google Scholar
28. Lagging, M., Romero, A. I., Westin, J., Norkrans, G., Dhillon, A. P., Pawlotsky, J. M., Zeuzem, S., von Wagner, M., Negro, F., Schalm, S. W., Haagmans, B. L., Ferrari, C., Missale, G., Neumann, A. U., Hart, E. V. & Hellstrand, K.(2006). “IP-10 Predicts Viral Response and Therapeutic Outcome in Difficult-to-Treat Patients With HCV Genotype 1 Infection,” Hepatology, 44(6), Pp. 1617-1625.
Publisher – Google Scholar
29. Lindh, M., Lagging, M., Farkkila, M., Langeland, N., Mørch, K., Nilsson, S., Norkrans, G., Pedersen, C., Buhl, M. R., Westin, J. & Hellstrand K. (2011). “Interleukin 28B Gene Variation at rs12979860 Determines Early Viral Kinetics During Treatment in Patients Carrying Genotypes 2 or 3 of Hepatitis C Virus,” Journal of Infectious Diseases, 203, Pp. 1748—1752.
Publisher – Google Scholar
30. McHutchison, J. G., Lawitz, E. J., Shiffman, M. L., Muir, A. J., Galler, G. W., McCone, J., Nyberg, L. M., Lee, W. M., Ghalib, R. H., Schiff, E. R., Galati, J. S., Bacon, B. R., Davis, M. N., Mukhopadhyay, P., Koury, K., Noviello, S., Pedicone, L. D., Brass, C. A., Albrecht, J. K. & Sulkowski, M. S. (2009). “Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection,” New England Journal of Medicine, 361, Pp. 580-593.
Publisher – Google Scholar
31. Payer, B. A., Reiberger, T., Aberle, J., Ferenci, P., Holzmann, H., Rieger, A. & Peck-Radosavljevic, M. (2012). “IL28B and interferon-gamma inducible protein 10 for prediction of rapid virologic response and sustained virologic response in HIV-HCV-coinfected patients,” European Journal of Clinical Investigation, 42(6), Pp. 599-606.
Publisher – Google Scholar
32. Suzuki, F., Suzuki, Y., Akuta, N., Sezaki, H., Hirakawa, M., Kawamura, Y., Hosaka, T., Kobayashi, M., Saito, S., Arase, Y., Ikeda, K., Kobayashi, M., Chayama, K., Kamatani, N., Nakamura, Y., Miyakawa, Y. & Kumada, H. (2011). “Influence of ITPA Polymorphisms on Decreases of Hemoglobin During Treatment with Pegylated Interferon, Ribavirin, and Telaprevir,” Hepatology, 53(2), Pp. 415-421.
Publisher – Google Scholar
33. Miyamura, T., Kanda, T., Nakamoto, S., Wu, S., Jiang, X., Arai, M., Fujiwara, K., Imazeki, F. & Yokosuka, O. (2012). “Roles of ITPA and IL28B Genotypes in Chronic Hepatitis C Patients Treated with Peginterferon Plus Ribavirin,” Viruses, 4, Pp. 1264-1278.
Publisher – Google Scholar
34. Moghaddam, A., Melum, E., Reinton, N., Ring-Larsen, H., Verbaan, H., Bjøro, K. & Dalgard, O. (2011). “IL28B Genetic Variation and Treatment Response in Patients with Hepatitis C Virus Genotype 3 Infection,” Hepatology, 53(3), Pp. 746-754.
Publisher – Google Scholar
35. Ochi, H., Maekawa, T., Abe, H., Hayashida, Y., Nakano, R., Kubo, M., Tsunoda, T., Hayes, C. N., Kumada, H., Nakamura, Y. & Chayama, K. (2010). “ITPA Polymorphism Affects Ribavirin-Induced Anemia and Outcomes of Therapy–A Genome-Wide Study of Japanese HCV Virus Patients,” Gastroenterology, 139, Pp. 1190—1197.
Publisher – Google Scholar
36. Petit, J. M., Carrat, F., Duong, M., Halfon, P., Duvillard, L., Bani-Sadr, F., Chavanet, P., Cacoub, P. & Piroth, L. (2010). “Response to anti-HCV therapy in HIV-HCV-coinfected patients: does the lipid profile really have an effect?,” Antiviral Therapy, 15, Pp. 797-800.
Publisher – Google Scholar
37. Poordad, F. (2011a). “IL28B Polymorphism Predicts Virologic Response In Patients With Hepatitis C Genotype 1 Treated With Boceprevir Combination Therapy,” Conference Proceedings of 46th Annual Meeting of the European Association for the Study of Liver Diseases (EASL 2011), Abstract 12, Berlin, 30 March — 3 April 2011.
38. Poordad, F., McCone, J. Jr., Bacon, B. R., Bruno, S., Manns, M. P., Sulkowski, M. S., Jacobson, I. M., Reddy, K. R., Goodman, Z. D., Boparai, N., DiNubile, M. J., Sniukiene, V., Brass, C. A., Albrecht, J. K., & Bronowicki, J. P. (2011b). “Boceprevir for untreated chronic HCV genotype 1 infection,” New England Journal of Medicine, 364, Pp. 1195—1206.
Publisher – Google Scholar
39. Ramcharran, D., Wahed, A. S., Conjeevaram, H. S., Evans, R. W., Wang, T., Belle, S. H. & Yee, L. J. (2010). “Associations between serum lipids and hepatitis C antiviral treatment efficacy,” Hepatology, 52(3), Pp. 854—863.
Publisher – Google Scholar
40. Rauch, A., Kutalik, Z., Descombes P, Cai, T., Di, L. J., Mueller, T., Bochud, M., Battegay, M., Bernasconi, E., Borovicka, J., Colombo, S., Cerny, A., Dufour, J. F., Furrer, H., Günthard, H. F., Heim, M., Hirschel, B., Malinverni, R., Moradpour, D., Müllhaupt, B., Witteck, A., Beckmann, J. S., Berg, T., Bergmann, S., Negro, F., Telenti, A., & Bochud, P. Y. (2010). “Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study,” Gastroenterology, 138(4), Pp. 1338—1345.
Publisher – Google Scholar
41. Reiberger, T., Aberle, J. H., Kundi, M., Kohrgruber, N., Rieger, A., Gangl, A., Holzmann, H. & Peck-Radosavljevic, M. (2008). “IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection,” Antiviral Therapy, 13(8), Pp. 969-976.
Google Scholar
42. Romero, A. I., Lagging, M., Westin, J., Dhillon, A. P., Dustin, L. B., Pawlotsky, J. M., Neumann, A. U., Ferrari, C., Missale, G., Haagmans, B. L., Schalm, S. W., Zeuzem, S., Negro, F., Hart, E. V. & Hellstrand, K. (2006). “Interferon (IFN)—g—Inducible Protein—10: Association with Histological Results, Viral Kinetics, and Outcome during Treatment with Pegylated IFN-a2a and Ribavirin for Chronic Hepatitis C Virus Infection,” Journal of Infectious Diseases, 194, Pp. 895—903.
Publisher – Google Scholar
43. Sarrazin, C., Susser, S., Doehring, A., Lange, C. M., Müller, T., Schlecker, C., Herrmann, E., Lötsch, J. & Berg, T. (2011). “Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients,” Journal of Hepatology, 54, Pp. 415—421.
Publisher – Google Scholar
44. Seto, W, K,, Tsang, O. T., Liu, K., Chan, J. M., Wong, D. K., Fung, J., Lai, C. L., Yuen, M. F. (2013). “Role of IL28B and inosine triphosphatase polymorphisms in the treatment of chronic hepatitis C virus genotype 6 infection,” Journal of Viral Hepatitis, 20(7), Pp. 470-477.
Publisher – Google Scholar
45. Shi, X., Pan, Y., Wang, M., Wang, D., Li, W., Jiang, T., Zhang, P., Chi, X., Jiang, Y., Gao, Y., Zhong, J., Sun, B., Xu, D., Jiang, J. & Niuet, J. (2012). “IL28B Genetic Variation Is Associated with Spontaneous Clearance of Hepatitis C Virus, Treatment Response, Serum IL-28B Levels in Chinese Population,” PLoS ONE, 7(5), Pp. e37054.
Publisher – Google Scholar
46. Soriano, V., Poveda, E., Vispo, E., Labarga, P., Rallon, N. & Barreiro, P. (2012). “Pharmacogenetics of hepatitis C,” Journal of Antimicrobial Chemotherapy, 67(3), Pp. 523-529.
Publisher – Google Scholar
47. Suppiah, V., Moldovan, M., Ahlenstiel, G., Berg, T., Weltman, M., Abate, M. L., Bassendine, M., Spengler, U., Dore, G. J., Powell, E., Riordan, S., Sheridan, D., Smedile, A., Fragomeli, V., Müller, T., Bahlo, M., Stewart, G. J., Booth, D. R., & George, J. (2009). “IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy,” Nature Genetics, 41(10), Pp. 1100—1104.
Publisher – Google Scholar
48. Tanaka, Y., Nishida, N., Sugiyama, M., Kurosaki, M., Matsuura, K., Sakamoto, N., Nakagawa, M., Korenaga, M., Hino, K., Hige, S., Ito, Y., Mita, E., Tanaka, E., Mochida, S., Murawaki, Y., Honda, M., Sakai, A., Hiasa, Y., Nishiguchi, S., Koike, A., Sakaida, I., Imamura, M., Ito, K., Yano, K., Masaki, N., Sugauchi, F., Izumi, N., Tokunaga, K., & Mizokami, M. (2009). “Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C,” Nature Genetics, 41(10), Pp. 1105—1109.
Publisher – Google Scholar
49. Tanaka, Y., Kurosaki, M., Nishida, N., Sugiyama, M., Matsuura, K., Sakamoto, N., Enomoto, N., Yatsuhashi, H., Nishiguchi, S., Hino, K., Hige, S., Itoh, Y., Tanaka, E., Mochida, S., Honda, M., Hiasa, Y., Koike, A., Sugauchi, F., Kaneko, S., Izumi, N., Tokunaga, K. & Mizokami, M. (2011). “Genome-wide association study identiï¬ed ITPA/DDRGK1 variants reflecting thrombocytopenia in pegylated interferon and ribavirin therapy for chronic hepatitis C,” Human Molecular Genetics, 20(17), Pp. 3507-3516.
Publisher – Google Scholar
50. Thompson, A. J., Fellay, J., Patel, K., Tillmann, H. L., Naggie, S., Ge, D., Urban, T. J., Shianna, K. V., Muir, A. J., Fried, M. W., Afdhal, N. H., Goldstein, D. B. & Mchutchison, J. G. (2010). “Variants in the ITPA Gene Protect Against Ribavirin-Induced Hemolytic Anemia and Decrease the Need for Ribavirin Dose Reduction,” Gastroenterology, 139(4), Pp. 1181—1189.
Publisher – Google Scholar
51. Thorlund, K., Druyts, E., Khoury, A. C. E. & Mills, E. J. (2012). “Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection,” Clinico Economics and Outcomes Research, 4, Pp. 349—359.
Google Scholar
52. U.S. Food and Drugs Administration. (2014). “Olysio (simeprevir) for the treatment of chronic hepatitis C in combination antiviral treatment,” U.S. Food and Drugs Administration, for Consumers. [ONLINE], [Retrieved March 9, 2014], http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm377234.htm
53. Vutien, P., Nguyen, N. H., Trinh, H. N., Li, J., Garcia, R. T., Garcia, G., Nguyen, K. K., Nguyen, H. A., Levitt, B. S., Keeffe, E. B. & Nguyen, M. H. (2010). “Similar Treatment Response to Peginterferon and Ribavirin in Asian and Caucasian Patients with Chronic Hepatitis C,” American Journal of Gastroenterology, 105, Pp. 1110-1115.
54. Welsch, C., Jesudian, A., Zeuzem, S. & Jacobson, I. (2012). “New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives,” Gut, 61(Suppl 1), Pp. i36-i46.
Publisher – Google Scholar
55. Zeuzem, S., Andreone, P., Pol, S., Lawitz, E., Diago, M., Roberts, S., Focaccia, R., Younossi, Z., Foster, G. R., Horban, A., Ferenci, P., Nevens, F., Müllhaupt, B., Pockros, P., Terg, R., Shouval, D., van Hoek, B., Weiland, O., Heeswijk, R. V., Meyer, S. D., Luo, D., Boogaerts, G., Polo, R., Picchio, G., & Beumont, M. (2011). “Telaprevir for retreatment of HCV infection,” New England Journal of Medicine, 364, Pp. 2417—2428.
Publisher – Google Scholar