Discussion
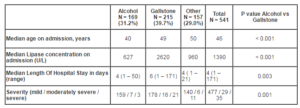
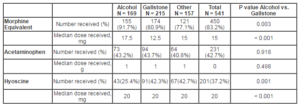
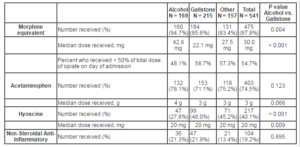
Our study demonstrates that a wide variety of analgesics are prescribed for patients with acute pancreatitis. Opioids constitute the main analgesic — administered during 87.8% episodes of care. Acetaminophen (paracetamol) is underutilised especially on the day of admission but even by the end of day 3, one quarter of patients had not received this opiate sparing analgesic. NSAID are rarely administered within the first three days (< 20% of admissions). Surprisingly, hyoscine was administered during 40% of admissions. Around 50% of patients received at least half their three day opiate dose on the day of admission. Not only were patients with alcohol-related AP more likely to receive opiate analgesia than those with gallstone disease, they also received a higher dose (median 42.8mg vs. 22.1mg over three days; p < 0.001). Pain level on admission was recorded for only 44.0% of presentations and this may explain the finding that pain level was not related to analgesia requirement, etiology of pancreatitis, lipase concentration and the development of severe pancreatitis. This is the first study to our knowledge to demonstrate that the lipase concentration on admission is of no use in determining the patient’s opiate analgesic requirement.
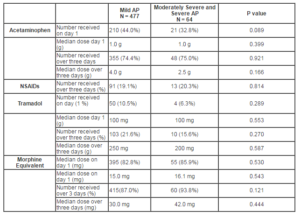
Our data show no difference in the use of analgesics or dose given on day 1 and up to day 3 in patients with mild and non-mild AP. However, an Italian audit found patients with mild AP were more likely to receive NSAIDs and Tramadol than those with severe AP, where as the opposite was true for opioids (Pezzilli et al., 2007). It may be extrapolated that these differences in analgesia requirements arise after the third day of admission.
The high rate of opiate use reflects the potency of this class of analgesic and is not unexpected (Basurto et al., 2013). It is unclear why around 1 in 10 patients received no opiate over the first three days – possibly the pain had spontaneously resolved on presentation or it is a demonstration that clinicians sometimes provide inadequate analgesia. The underutilization of acetaminophen (paracetamol) and NSAIDs may reflect the scarcity of guidelines and poor acceptance of the concept of multi-modal analgesia. The low rate of use may be contributed to by patients being nil by mouth (possibly arbitrarily as in the traditional approach to managing patients with AP; perhaps due to vomiting or while awaiting imaging).
The evidence base on how best to relieve pain in those with acute pancreatitis is limited. Not only is there a scarcity of studies, but many are of low quality (Meng et al., 2013), and only five were suitable for a recent Cochrane review (Basurto et al., 2013). Not surprisingly therefore, only three of the nine recent guidelines mention analgesia (Takeda et al., 2006, Toouli et al., 2002, Pezzilli et al., 2008) — the first of which (from Japan) recommends NSAID use in mild AP. The Italian position statement recommends graded prescription of analgesia depending on the severity of pain (Pezzilli et al., 2008). The morphine-sparing ability of indomethacin in AP is long known (Ebbehøj et al., 1985). Acetaminophen (paracetamol) is recognized for its safety, effectiveness as an analgesic as well as its opioid-sparing effects (Remy et al., 2005). None of our patients were treated with buprenorphine, even though two randomized trials have demonstrated advantages to its use (Blamey et al., 1984, Jakobs et al., 2000). Pancreatic enzyme supplementation has been shown not to reduce pain scores in AP (Patankar et al., 1995). A recent review of hyoscine does not recommend the agent for patients with AP (Tytgat, 2007), and it is quite surprising this antispasmodic was administered to so many of our patients. However, hyoscine may have a role in managing biliary colic (Kumar et al., 2004). The fact that morphine constitutes the principal opiate in our series suggests resolution of the morphine and meperidine (pethidine) debate (at least in our hospital). Katerndahl traces the concept of morphine-induced sphincter of Oddi (SO) spasm to a 1965 textbook of Surgery by Bailey and Love (Spiegel, 2001). There is agreement that in patients without acute pancreatitis, at low dose, morphine increases wave frequency to a greater extent than pethidine but neither affects the SO basal pressure (Isenhower and Mueller, 1998, Thompson, 2001). At higher cumulative doses, morphine increases the basal pressure of the sphincter while pethidine does not (Isenhower and Mueller, 1998). While both are potent opioids, the metabolites of Meperidine (pethidine) accumulate in patients with renal impairment and may cause seizures. Meperidine (pethidine) also crosses the blood-brain barrier resulting in euphoria. Tramadol hydrochloride has been associated with seizures (Raiger et al., 2012). There is no evidence on the effects of these two opioids on SO in patients with AP.
The strengths of this study include the following: diagnosis confirmed by clinician rather than relying on administrative coding data which is prone to error (Zhan and Miller, 2003), large number of patients, relatively long time frame i.e. not just the first 24 hours. A major limitation of the study is the low frequency of documented pain levels on admission and the study’s non-randomized design. In addition, the ME opiate dose may constitute an over simplification, as each of the medications has a different side effect profile. Timing of oral refeeding was not analyzed as those without a clear alcohol etiology were routinely fasted in preparation for abdominal ultrasonography. A small proportion of admissions with clinically acute pancreatitis due to alcohol may have underlying chronic pancreatitis, and this may have affected the analgesics prescribed. Future research may include a greater emphasis on multimodal analgesia, evaluation of the role of hyoscine or newer non-constipating analgesics such as slow release oxycodone with naltrexone (naloxone) (Davis et al., 2013).
The clinical implications of our study fall into two domains. As would be expected, opioids should remain the principal analgesic when patients with AP are initially assessed in the Emergency Department, but we found no evidence to support the ongoing use of hyoscine. Once the patient is admitted to the acute ward, clinicians could consider prescribing acetaminophen and Non-Steroidal Anti-Inflammatory medications, in addition to opioids, as part of a multimodal analgesia strategy.
Opioids are the main analgesic in patients with acute pancreatitis. Uniquely, this study demonstrates: 1) patients with alcohol-related AP require a higher dose of opioids than those with biliary AP; 2) lipase concentration on admission is of no use in determining the patient’s opiate analgesic requirement, and 3) hyoscine is unhelpful in AP. We would suggest future guidelines on the management of patients with acute pancreatitis specifically mention analgesia, otherwise the analgesic pyramid tends to be forgotten and the wheel is re-invented each time a patient with acute pancreatitis is admitted.
Abbreviations
AP acute pancreatitis; ME morphine equivalent; NSAID Non-Steroidal Anti-Inflammatory; SO Sphincter of Oddi
Acknowledgements
Mr. James Hogan for providing statistical analysis. WiltshireHogan Limited. 91b Normandale Road, Normandale, Lower Hutt, New Zealand.
References
1. BANKS, P. A., BOLLEN, T. L., DERVENIS, C., GOOSZEN, H. G., JOHNSON, C. D., SARR, M. G., TSIOTOS, G. G. & VEGE, S. S. 2013. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut, 62, 102-11.
Publisher – Google Scholar
2. BASURTO, O. X., RIGAU, C. D. & URRUTIA, G. 2013. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev, 7, CD009179.
Google Scholar
3. BLAMEY, S. L., FINLAY, I. G., CARTER, D. C. & IMRIE, C. W. 1984. Analgesia in acute pancreatitis: comparison of buprenorphine and pethidine. Br Med J (Clin Res Ed), 288, 1494-5.
Publisher – Google Scholar
4. BUTER, A., IMRIE, C. W., CARTER, C. R., EVANS, S. & MCKAY, C. J. 2002. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. British Journal of Surgery., 89, 298-302.
Publisher – Google Scholar
5. DAVIS, M., GOFORTH, H. W. & GAMIER, P. 2013. Oxycodone combined with opioid receptor antagonists: efficacy and safety. Expert Opin Drug Saf, 12, 389-402.
Publisher – Google Scholar
6. EBBEHØJ, N., FRIIS, J., SVENDSEN, L. B., BULOW, S. & MADSEN, P. 1985. Indomethacin treatment of acute pancreatitis. A controlled double-blind trial. Scand J Gastroenterol, 20, 798-800.
Publisher – Google Scholar
7. ISENHOWER, H. L. & MUELLER, B. A. 1998. Selection of narcotic analgesics for pain associated with pancreatitis. Am J Health Syst Pharm, 55, 480-6.
Google Scholar
8. JAKOBS, R., ADAMEK, M. U., VON BUBNOFF, A. C. & RIEMANN, J. F. 2000. Buprenorphine or procaine for pain relief in acute pancreatitis. A prospective randomized study. Scand J Gastroenterol, 35, 1319-23.
Publisher – Google Scholar
9. KOIZUMI, M., TAKADA, T., KAWARADA, Y., HIRATA, K., MAYUMI, T., YOSHIDA, M., SEKIMOTO, M., HIROTA, M., KIMURA, Y., TAKEDA, K., ISAJI, S., OTSUKI, M. & MATSUNO, S. 2006. JPN Guidelines for the management of acute pancreatitis: diagnostic criteria for acute pancreatitis. J Hepatobiliary Pancreat Surg, 13, 25-32.
Publisher – Google Scholar
10. KUMAR, A., DEED, J. S., BHASIN, B. & THOMAS, S. 2004. Comparison of the effect of diclofenac with hyoscine-N-butylbromide in the symptomatic treatment of acute biliary colic. ANZ J Surg, 74, 573-6.
Publisher – Google Scholar
11. MENG, W., YUAN, J., ZHANG, C., BAI, Z., ZHOU, W., YAN, J. & LI, X. 2013. Parenteral analgesics for pain relief in acute pancreatitis: a systematic review. Pancreatology, 13, 201-6.
Publisher – Google Scholar
12. PATANKAR, R. V., CHAND, R. & JOHNSON, C. D. 1995. Pancreatic enzyme supplementation in acute pancreatitis. HPB Surg, 8, 159-62.
Publisher – Google Scholar
13. PEZZILLI, R., UOMO, G., GABBRIELLI, A., ZERBI, A., FRULLONI, L., DE RAI, P., CASTOLDI, L., CAVALLINI, G. & DI CARLO, V. 2007. A prospective multicentre survey on the treatment of acute pancreatitis in Italy. Dig Liver Dis, 39, 838-46.
Publisher – Google Scholar
14. PEZZILLI, R., UOMO, G., ZERBI, A., GABBRIELLI, A., FRULLONI, L., DE RAI, P., DELLE FAVE, G. & DI CARLO, V. 2008. Diagnosis and treatment of acute pancreatitis: the position statement of the Italian Association for the study of the pancreas. Dig Liver Dis, 40, 803-8.
Publisher – Google Scholar
15. RAIGER, L. K., NAITHANI, U., BHATIA, S. & CHAUHAN, S. S. 2012. Seizures after intravenous tramadol given as premedication. Indian J Anaesth, 56, 55-7.
Publisher – Google Scholar
16. REMY, C., MARRET, E. & BONNET, F. 2005. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth, 94, 505-13.
Publisher – Google Scholar
17. ROYAL AUSTRALASIAN COLLEGE OF GENERAL PRACTICIONERS. 2012. Pain Management [Online]. Royal Australasian College of General Practicioners. Available: http://www.racgp.org.au/your-practice/guidelines/silverbook/common-clinical-conditions/pain-management/ [Accessed 27/05/2013 2013].
18. SPIEGEL, B. 2001. Meperidine or morphine in acute pancreatitis? Am Fam Physician, 64, 219-20.
19. TAKEDA, K., TAKADA, T., KAWARADA, Y., HIRATA, K., MAYUMI, T., YOSHIDA, M., SEKIMOTO, M., HIROTA, M., KIMURA, Y., ISAJI, S., KOIZUMI, M., OTSUKI, M. & MATSUNO, S. 2006. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J Hepatobiliary Pancreat Surg, 13, 42-7.
Publisher – Google Scholar
20. THOMPSON, D. R. 2001. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol, 96, 1266-72.
Publisher – Google Scholar
21. TOOULI, J., BROOKE-SMITH, M., BASSI, C., CARR-LOCKE, D., TELFORD, J., FREENY, P., IMRIE, C. & TANDON, R. 2002. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol, 17 Suppl, S15-39.
Publisher – Google Scholar
22. TYTGAT, G. N. 2007. Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs, 67, 1343-57.
Publisher – Google Scholar
23. WYSOCKI, A. P. & CARTER, C. R. 2007. Acute pancreatitis. Surgery, 25, 49-56.
24. ZHAN, C. & MILLER, M. R. 2003. Administrative data based patient safety research: a critical review. Qual Saf Health Care, 12 Suppl 2, ii58-63.
Publisher – Google Scholar