Introduction
Fertility declines significantly in recent years according to Rostand et al. (2013). Basic research helps to define genes and mechanisms responsible for development, function and regulation of the human reproductive system. However, a considerable amount of infertile couples are assigned to the category of idiopathic infertility, which is often bypassed with assisted reproductive technologies (ART) at the expense of high costs and accompanied by safety or ethical concerns. One of the most common causes of infertility or recurrent spontaneous abortions (RSA) is the genetical reason from which the chromosomal abnormalities take an important place. Among them the low-level gonosomal mosaicism (LLGM) (minority mosaic of hypo- or hyperdiploid X chromosome) shows a high incidence in females with reproductive impairment as it was published in papers written by Horsman et al. (1987), Gekas et al. (2001), Morel et al. (2004), Montag et al. (1997), Meschede et al. (1998), Mau et al. (1997), Čapkova et al. ((2004), Gekas et al. (2001), Scholtes et al. (1998), Sonntag et al. (2001), Peschka et al. (1999). Its significance has not been fully explained firstly due to the heterogeneity of LLGM definition used by various authors, secondly because the X aneuploidy shows a strong correlation with the age of females which means that a different age group has a different risk of reproductive problems as was published previously by Ford and Russell (1985), Guttenbach et al. (1995), Nowinski et al. (1990), Russell et al. (2007). Finally, different methodologies described in various studies make the final comparison of the results difficult. Toncheva et al. (1994) mentioned that clarifying the phenotypic relationships of chromosomal mosaicism is an important issue in cytogenetic diagnosis and genetic counselling. Studies performed by Sonntag et al. (2001) and Voight et al. (2004) confirmed that the LLGM does not show any impact on the outcome of IVF. Despite this fact, it has so far been unclear why the frequency of X- aneuploidy cells is so high in the population of females with reproductive failure. Ploidy of the X chromosome in the lymphocytes is mostly studied by the conventional cytogenetic method. However, this method is not optimal: the number of evaluated lymphocytes is limited, and only dividing cells are included in the assessment. The molecular-cytogenetic method (FISH) provides us with the possibility to detect gonosomal constitution also in interphase nuclei of uncultured lymphocytes, and it is the most appropriate method of confirming suspected mosaicism. This technique can also eliminate problems of the presence of culture artefacts. A similar method was used previously in the study of Patton et al. (2010). The aim of the study was to compare the incidence of the LLGM (and X aneuploidy cell proportion) in a group of female patients treated for infertility and the control cohort by the conventional method and by fluorescent in situ hybridization (FISH) in cultured and uncultured samples of peripheral blood. Correlation with age of females and relevant clinical data are included.
Patients and Methods
Cytogenetic Study
Routine cytogenetic analysis and FISH analysis were done in the group of 70 females, who were scheduled for IVF treatment in the Centre of Assisted Reproduction (median age = 30; range 19 – 42).The control group consisted of 83 females with at least two spontaneous conceptions without miscarriage history (median age = 32,5; range 22 – 46). The females were recruited mostly from the patients of the Department of Clinical Genetics and Foetal Medicine, where the blood sampling was taken as part of biochemical screening of pregnant females. All patients signed the informed consent. Confirmed fertility was defined as at least one natural pregnancy, no history of recurrent miscarriages, or any fertility treatments.
Cultured preparations were processed using standard cytogenetic procedures and evaluation and number scored metaphases respected “ECA Cytogenetic guidelines”. 15 – 20 metaphases were analyzed; the number of analyzed metaphases was increased at min 30 cells – appropriate confidence limits using Hook´s tables (1977) for lymphocyte cultures– when single cell aneuploidy was detected in the sample. Gonosomal mosaicism was defined as low-level (LLGM) when at least two or more of the analyzed cells but less than 20 % displayed the constitution 45,X or 47,XXX or X polysomy.
Every single cell aneuploidy was counted irrespective of whether the sample fulfilled the criteria of mosaicism to assess the frequency of X aneuploidy (45,X, 47,XXX, X polysomy) cells in both cohorts.
FISH study
For FISH we used X specific centromere probes (DXZ1) from Qbiogene (Heidelberg, Germany). Procedures were carried out according to the manufacturer´s recommendation. FISH was successfully performed on cultured lymphocytes in the test group of 66 females and 51 females of the control group. Interphase FISH was successfully performed on cultured lymphocytes in the test group of 54 females and 46 females of the control group. Uncultured blood was treated directly with hypotonic solution, 0.075 mol/l KCL, before fixing in methanol: acetic acid (3 : 1). At least 100 cells were scored in FISH tests (mean=170).
Clinical Data
The data of the control cohort were used for calculation of the “cutoff value” in FISH method. The cutoff (value discriminating between control and pathological level) was calculated separately for cultured (7.78 %) and uncultured blood (6.81 %) respectively as the mean plus 1.96 SD as was described previously by Kuo and Guo (2004). The cohort of 110 infertile females treated by ICSI in the Centre of Assisted Reproduction was divided into three groups according to the detection of LLGM without detected mosaic – control (N=33) or with mosaic either proved by standard cytogenetic method (N=44) or FISH with the cutoff value (N=33). The following parameters were assessed: previous pregnancies; previous spontaneous abortions; recurrent spontaneous abortions; ICSI cycles/patient; number of oocytes retrieved; fertilisation rate.
Statistics
Results between the test and control group were tested for significance using the Mann-Whitney U-test and the chi-square test as appropriate. A p-value of p ˂ 0.05 was considered to show a significant difference.
Results
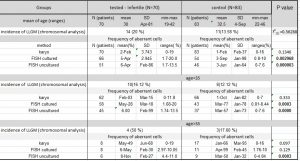
The incidence of LLGM (at least two cells in sample show X aneuploidy) in the group of 70 patients was 14 (20 %) vs.11 (13.58 %) in the control group of 83 healthy females (p>0.05). X chromosome loss was detected in 10 %; X chromosome gain in 2.85 % and presence of the both cell lines in 7.15 % of patients vs. 8.64 %females with X loss and 4.94 % females with both of the cell lines in the control.
The mean of proportion of X aneuploidy cells was 2.2 % (SD=3,743; R 0-19) in the tested group vs 1.2 % (SD 2,37; R 0-16) in the control group by cytogenetic examination. Although the frequency of aberrant cells was higher in the group of infertile females, a significant difference was not confirmed (p >0.05). The mean of proportion of X aneuploidy cells was 5,4 % (SD=2.945; R 1,7-20) in the infertile group vs. 3.9 % (SD=1.98; RI 0.8-10) in the control group of cultured samples tested by FISH (p<0.05). The mean of proportion of X aneuploidy cells was 6,4 % (SD=2.86; R 1.7-13.5) in the infertile group vs. 3.6 % (SD=1.64; R 0-7.6) in the control group of uncultured samples tested by FISH (p<0.05).The frequency of X aneuploidy cells was significantly higher in the infertile group than in the control group for cultured and uncultured samples tested by FISH (tab. 1, fig. 1).
The frequency of aberrant cells similarly as the incidence of LLGM in females increases with age (fig. 2). The differences between particular age groups were not significant.
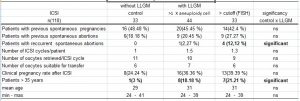
Analysis of clinical data shows a significant difference between the incidence of RSA in the patients without LLGM (0 %) and those who have LLGM detected with FISH 4 (12,12 %)(P˂0.05). In the LLGM group is the highest occurrence of females older than 35 (tab. 2).
Discussion
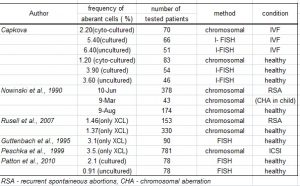
The range of detected LLGM in the group of female patients with reproductive failure varies from 2,77 % to 16 % in so far published studies ( see tab. 3a). LLGM is the predominant chromosomal aberration in this group of females. However, it is also detected in females with normal reproductive capacity as it was published previously by Horsman et al. (1987). There was a significant difference in the frequency of X aneuploidy cells in the group of females treated for infertility vs. control group by FISH in cultured and uncultured samples in our study. The cutoff values for cultured and uncultured cells were 7.78 % and 6.81 % resp. The frequency of sex aneuploidy from 6 % showed significant relevance in the study of Homer et al. (2010).
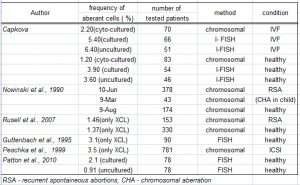
The majority of studies – Peschka et al. (1999), Nowinski et al. ((1990), Voight et al. (2004) – are based on cytogenetic analysis and clinical data are related to the incidence of LLGM (defined as at least two aberrant cells in the sample) in female. Voight et al. (2004), Sonntag et al. (2001) and Meschede et al. (1998) used for determining of the significance of the low-level gonosomal aneuploidy 15, 10 and 6 % respectively cutoff for LLGM. These results are biased by number of checked cells, culture artefacts and different cutoff values used by these authors. Moreover, the age of females was not taken into account. Scoring of all X aneuploidy cells irrespective of LLGM definition was published by Ford and Russell (1985), Nowinski et al. (1990) and Kuo and Guo (2004) in studies of LLGM and age correlation (sees tab. 3b). FISH has not been used by many authors previously, and comparison to the interval of referral values is essential. Patton et al. (2010) detected the frequency of aberrant cells in the group of fertile females lower than 5 % by FISH. Similar results were obtained in our study in the control cohort.
The correlation between infertility and LLGM has not yet been confirmed. Sonntag et al. (2001) and Voight et al. (2004) proved that this chromosomal aberration is without any influence on the outcome of IVF in females treated for infertility. However, the results of studies written by Homer et al. (2010), Kuo and Guo (2004) and Voight et al. (2004) imply that the patients with LLGM show higher predisposition to spontaneous abortions. Our data lead to the same conclusion (frequency of RSA 12,12 % with LLGM vs 0 % without LLGM). Magli et al. (2002) suggests that the constitutional carriers of sex chromosomal mosaicism are predisposed to autosomal mosaicism in the embryo due to the probable inborn mitotic error of cell division which has a higher tendency in the female than in the male. Kovaleva et al. (2005) published two mechanisms that contribute to women´s susceptibility to minor mosaicism: sex-specific cell loss and sex-specific centromere instability. The occurrence of LLGM is age dependent. Fitzgerald (1983) described that the X hypoploidy (strongly associated with age) occurs mostly as a result of premature centromere division. Our clinical data imply that higher frequency of X aneuploidy cells is mostly influenced by the age of the patient. There were significantly more patients aged above 35 years in the groups with LLGM (proved either cytogenetically or FISH) compared to the group without LLGM in the clinical data assessment. The frequency of X aneuploidy cells was increased in age group above 35 years in comparison to younger (below 35) one but not significantly. Guttenbach et al. (1995) found a significant correlation only in women aged above 51. The frequency of X aneuploidy cells in particular age groups in the tested cohort resembles the frequency of X aneuploidy cells in fertile females of the older age group. This finding suggests that in females with LLGM errors of cell division occur more often than in females with normal reproductive capacity. If the malsegregation of chromosomes is associated with aging than we might speculate that the chronological age of females treated for infertility might differ from biological one and then these patients are more prone to acceleration of cell senescence. A similar idea was presented in the studies of Magli et al. (2002) and Nowinski et al. (1990). This hypothesis is also supported by the fact that there is a relatively high proportion of single cell aneuploidy of different chromosomes in females treated for infertility that was previously published by Peschka et al. (1999). Leach et al. confirmed by study of telomere length that acquired somatic cell aneuploidy is associated with aging. The association of age and infertility and age and pregnancy loss were confirmed by Grande et al. (2012). The authors of so far published studies have not been yet consistent in the correlation between LLGM and pregnancy loss.
The highest frequency of RSA was detected in the group of females treated for infertility with the X aneuploidy cells proportion higher than the cutoff. Despite that the mean age (31) in this group did not significantly differ from the other subgroups there was the highest rate of women aged above 35 (21 %)(tab. 2). It might suggest that females with LLGM are prone to recurrent abortions, and the LLGM could be a marker for biological age of patients. In a study by Devi et al. (1998) the higher incidence of LLGM is in patients with idiopathic premature ovarian failure. Kuo and Guo (2004) also imply the possible presence of diminished ovarian reserve in the presence of LLGM, which is associated with the spontaneous abortions or a higher rate of aneuploidy in foetuses. On the contrary according to study of Homer et al. (2010) the sex chromosome mosaicism below 30 % has no impact on the ovarian reserve of females. However, his study confirmed significantly higher rate of RSA in the group of patient with LLGM below 30 %.
Conclusion
The significant difference in frequency of X aneuploidy cells was confirmed between tested and control cohort by FISH. However, outcome of IVF was not significantly different in patients with LLGM from the patients without this chromosomal aberration. Females with LLGM detected by FISH showed significantly higher tendency to RSA, which appears to be influenced by age since there were significantly more patients older than 35 years in the group with LLGM detected by FISH than in the control. By using FISH, a large number of cells can be rapidly counted which facilitates further assessment of low-level aneuploidy. The calculation cutoff value for different methods, tissues and age group should be used. Detection of X aneuploidy proportion higher than the cutoff in patients should not be underestimated due to the possible presence of gonadal mosaicism in the patient and following consequences for pregnancy. Lymphocyte analysis is a standard cytogenetic practice however it cannot address the issue of tissue limited mosaicism, and thus it may not reflect the chromosomal constitution in gonads. The genetic counselling is recommended for the females with detected LLGM.
List of abbreviations:
IVF in vitro fertilisation
ICSI intracytoplasmatic sperm injection
RSA recurrent spontaneous abortions
LLGM low-level gonosomal mosaicism
FISH fluorescent in situ hybridization
Conflict of Interest statement The authors declare that there are no conflicts of interest.
Tab. 1 : Incidence of LLGM and frequency of X aneuploidy in both cohort
(significant p˂0.05). References
Tab. 2: Analysis of clinical data
Tab.3a: Incidence of LLGM in females – the literature overview
Tab.3b: Frequency of X aneuploidy cells in females – the literature overview
(adsbygoogle = window.adsbygoogle || []).push({});
References
- Capkova, P., Adamova, K., Santava, A., Braunerova, B., Kolarova, J., Polak, P., Sobek, A., Oborna, I., Santavy, J. (2004). “ The importance of genetic investigation in couples with the reproduction impairment.” Česká Gynekologie, 69(1), 66-71.
- Devi, A. S., Metzger, D. A., Luciano, A. A.,Benn, P. A. (1998)” 45,X/46,XX mosaicism in patients with idiopathic premature ovarian failure.” Fertility and Sterility, 70(1),89-93.
- Fitzgerald, P. H. (1983)” Premature centromere division of the X chromosome.” In Sanberg AA (ed.): Cytogenetics of Mammalian X Chromosome Part A, Basic Mechanism of X Chromosome Behaviour. N. Y.: Alan R. Liss, 171-184
- Ford, J. H. and Russell, J. A. (1985) “Differences in the error mechanisms affecting sex and autosomal chromosomes in women of different ages within the reproductive age group.” American Journal of Human Genetics, 37(5), 973-983.
-
- Gekas, J., Thepot, F., Turleau, C., Siffroi, J. P., Dadoune, j. P., Briault, S., Rio, M., Bourouillou, G., Carre-Pigeon, F., Wasels R., Benzacken, B. (2001)” Chromosomal factors of infertility in candidate couples for ICSI: an equal risk of constitutional aberrations in women and men.” Human Reproduction ,16(1):82-90
- Grande, M., Borell, A., Garcia-Posada, R.,Borobio, V., Muñoz, M., Montserrat, C., Soler, A., Sanchez, A., Balasch, J.(2012)” The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage.” Human Reproduction, 27(10), 3109-3117
- Guttenbach, M., Koschorz, B., Bernthaler, U., Grimm, T., Schmid, M. (1995) “Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei.” American Journal of Human Genetics, 57(5), 1143-50.
- Hook EB. (1977) „Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use.” American Journal of Human Genetics, Jan;29(1):94-7.
- Homer, L., Le Martelot, M. T., Morel, F.,Amice, V. Kerlan, V., Collet, M., De Braekeleer, M (2010)” 45,X/46,XX mosaicism below 30 % of aneuploidy: clinical implications in adult women from reproductive medicine unit.” European Journal of Endocrinology, 162(3), 617-623
- Horsman, D. E., Dill, F. J., McGilivray, B. C., Kalousek, D. K. (1987) „ X chromosome aneuploidy in lymphocyte culture from women with recurrent spontaneous abortion.” American Journal of Medical Genetics, 28(4), 981 –987.
- Kovaleva, N. V. (2005)” Sex-specific chromosome instability in early human development.” American Journal of Medical Genetics. 136A(4),401-13
- Kuo, P. L, Guo, H. R.(2004)” Mechanism of recurrent spontaneous abortions in women with mosaicism of X-chromosome aneuploidies.” Fertility and Sterility, 82(6), 1594-1601
- Leach, N. T., Rehder, C., Jensen, K., Holt, S., Jackso-Cook, C. (2004) “Human chromosomes with shorter telomeres and large heterochromatin regions have a higher frequency of acquired somatic cell aneuploidy.” Mechanism of Ageing and Development, 125(8), 563-573.
- Magli, M. C., Gianaroli, L., Ferraretti, A. P., Gordts, S., Feliciani, E. (2002) „Impact of parental gonosomal mosaicism detected in peripheral blood on preimplatation embryos.” Reproductive Biomedicine online, 5(3), 306-312.
- Mau, U. A., Bäckert, I. T., Kaiser, P., Kiesel, L. (1997) “Chromosomal findings in 150 couple referred to genetic counselling prior ICSI.” Human Reproduction, 12(5), 930-7.
- Meschede, D., Lemcke, B., Exeler, J. R., De Geyter, C., Behre, H. M., Nieschlaq, E., Horst, J.(1998) Chromosome abnormalities in 447 couples undergoing ICSI – prevalence, type, sex distribution and reproductive relevance. Human Reproduction, 13(3),576-82.
-
- Montag, M., Van der Ven, K., Ved, S., Schmutzler, A., Prietl, G. (1997)” Success of intracytoplasmic sperm injection in couples with male and/or female chromosome aberrations.” Human Reproduction ,12(12), 2635-40
- Morel, F., Douet-Guilbert, N., Le Bris, M. J., Amice, V., Le Merlot, M. T. (2004)” Chromosomal abnormalities in couples undergoing ICSI. A study of 370 couples and review of the literature” International Journal of Andrology, 27, 178-182.
- Nowinski, G. P., Van Dyke, D. L., Tilley, B. C., Jacobsen, G., Babu, V. R. (1990) “The frequency of aneuploidy in cultured lymphocytes is correlated with age and gender but not with reproductive history.” American Journal of Human Genetics, 46, 1101-1111.
- Patton, K., Murch, A., Goldblatt, J., Wetherall, J., Doherty, D., Hadlow N. (2010)” Rate of X chromosome aneuploidy in young fertile women: Comparison of cultured and uncultured cell preparation using FISH.” Australian and New Zealand Journal Obstetric and Gynaecology, 50(4): 378-381.
- Peschka, B., Leygraaf, J., Van der Ven, K., Montag, M. (1999) “Type and frequency of chromosome aberrations in 781 couples undergoing ICSI.” Human Reproduction, 14(9), 2257-2263.
- Rostand, B., Schmidt, L., Sundby, J., Schei, B. (2013)”Has fertility declined from mid-1990s to mid-2000s?” Acta Obstetricia et Gynecologica Scandinavica, 92(11), 1284-1289.
- Russell, L. M., Strike, P., Browne, C. E., Jacobs, P. A. (2007) “X chromosome loss and ageing.” Cytogenetic Genome Research, 116(3), 181-185
- Scholtes, M. C. W., Behrend, C., Dietzel-Dahmen, J., van Hoogstraten, D. G., Marx, K., Wohlers, S., Verhoeven, H., Zeilmaker, G. H. (1998) “Chromosomal aberration in couples undergoing intracytoplasmic sperm injection: influence on implantation and ongoing pregnancy rate.” Fertility and Sterility, 70(5), 933-37.
- Sonntag, B., Meschede, D., Ulmann, V., Gassner, P., Horst, J. (2001)” Low-level sex chromosome mosaicism in female partners of couples undergoing ICSI therapy does not significantly affect treatment outcome” Human Reproduction 1(8)6, 1648-1652.
- Toncheva, D., Ilieva, P., Mavrudieva, M. (1994)”Detection of low-level sex-chromosome mosaicism.” Genetic Counselling, 5(4),363-367.
- Voight, R., Schröder, A. K., Hinrichts, E., Diedrich,, K., Schwinger, E., Ludwig, M. (2004)” LLGM in women undergoing ICSI cycles.” Journal of Assisted Reproduction and Genetics,21(5), 149-55.






