Quantification of Methyl Esters
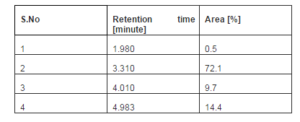
The amount of methyl esters in the product after transesterification of neem oil was analyzed using High Performance Liquid chromatography (HPLC) (SHIMADZU, JAPAN), as shown in Figure 4 and its quantification in percentage results are presented in Table 2. Neem oil contains mainly average compositions of four fatty acids like linoleic acid (6-16%), oleic acid (25-54%), hexadecanoic acid (16-33%) and octadecanoic acid (9-24%). There are four peaks have appeared in the HPLC spectrum which are indicating that the corresponding methyl esters of the fatty acids (Anya et al. 2012, p.21).
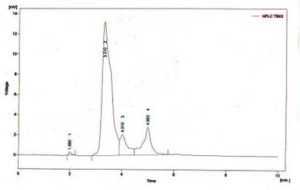
Fig. 4. HPLC Chromatogram of Neem Oil Methyl Esters
Table 2 .Quantification of Neem Oil Methyl Esters
Conclusion
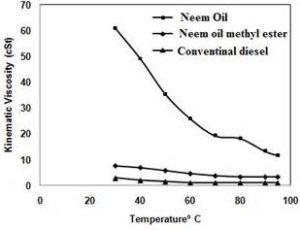
The production of biodiesel from the oil through two step processes like acid and alkaline transesterification method has been demonstrated. It was shown that the crude oil from neem is well suited for the production of biodiesel and it has comparable qualities of biodiesel. The advantage of this method is the lower reaction, temperature and duration of time are required. The maximum conversion of 85% was obtained at 60ºC with 300 ml methanol for NaOH (14.5g per liter of oil) catalyzed transesterification. The methyl ester fuel properties were found to be close to conventional diesel and compared to other methyl esters. The viscosity of neem oil biodiesel has 5.81 cSt, It is slightly higher than conventional diesel. The viscosity can be reduced by increasing the diesel content and temperature of the blend. Blends of 20% biodiesel with ordinary diesel are very close to conventional diesel and also the viscosity of neem oil biodiesel is close to conventional diesel at the temperature above 60ºC. The methyl esters of neem oil have identified by the HPLC spectrum which illustrated that four fatty acids of neem oil were converted into corresponding fatty acid methyl esters.
Acknowledgement
Financial support from Tamil Nadu State Council for Science and Technology (TNSCST), Chennai, TamilNadu, India is gratefully acknowledged.
References
Anya, A. U., Chioma, N. N. & Obinna, O. (2012). “Optimized Reduction of Free Fatty Acid Content on Neem Seed Oil for Biodiesel Production,” Journal of Basic and Applied Chemistry, Vol. 2, pp. 21-28.
Publisher – Google Scholar
Azam, M. M., Waris, A. & Nahar, N. M. (2005). “Prospects and Potential of Fatty Acid Methyl Esters of Some Non-Traditional Seed Oil for Use as Biodiesel in India,” Biomass & Bioenergy, vol.29, pp. 293-302.
Publisher – Google Scholar
Barnwal, B. K. & Sharma, M. P. (2005). “Prospects of Biodiesel Production from Vegetable Oils in India,” Renewable and Sustainable Energy Reviews, vol. 9, pp. 363-378.
Publisher – Google Scholar
Bozbas, K. (2005). “Review Biodiesel as an Alternative Motor Fuel: Production and Policies in the European Union,”Renewable & Sustainable Energy reviews, vol.2, 1-12.
Publisher – Google Scholar
Canakci, M. & Van Gerpen, J. (2001). “Biodiesel Production from Oils and Fats with High Free Fatty Acids,”Transactions of ASAE, vol.44, pp. 1429—1436.
Publisher – Google Scholar
Deepak, T., Ajayta, D. S. & Mathur, Y. P. (2013). “Production and Characterization of Neem Oil Methyl Ester,”International Journal of Engineering Research & Technology (IJERT), vol.2, pp.1896-1903.
Publisher
Demirbas, A. (2005). “Biodiesel Production from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Methanol Transesterification Methods,” Progress in Energy and Combustion Science, vol.31, pp. 466-487.
Publisher – Google Scholar
Dhar, A., Kevin, R. & Agarwal, A. K. (2012). “Production of Bidiesel from High-FFA Neem Oil and its Performance, Emission and Combustion Characterization in a Single Cylinder DICI Engine,” Fuel Processing Technology, vol.97, pp. 118-129.
Publisher – Google Scholar
Di Serio, M. Tesser, R., Pengmei, L. & Santacesaria, E. (2008). “Heterogeneous Catalysts for Biodiesel Production,”Energy & Fuels, vol.22, pp. 207-217.
Publisher – Google Scholar
Dorado, M. P., Ballesteros, E., Arnal, J. M., Gomez, J. & Lopez, F. J. (2003). “Exhaust Emissions from a Diesel Engine Fueled with Transesterified Waste Olive Oil,” Fuel, vol.82, pp. 1311-1315.
Publisher – Google Scholar
Elkadi, M., Pillay, A. E., Manuel, J., Stephan, S. & Khan, M. Z. (2013). “Kinetic Study of Neem Oil Biodiesel Production,” British Biotechnology Journal, vol.3 pp. 500-508.
Publisher
Foidl, N. G., Foidl, G., Sanchez, M., Mittelbach, M. & Hackel, S. (1996). “Jatropha Curcas L. As a Source for the Production of Biofuel in Nicaragua,” Bioresource Technology, vol.58, pp. 77-82.
Publisher – Google Scholar
Ghadge, S. V. & Raheman, H. (2006). “Process Optimization for Biodiesel Production from Mahua (Madhuca Indica) Oil Using Response Surface Methodology,” Bioresource Technology, vol. 97, pp. 379-384.
Publisher – Google Scholar
Karmee, S. K. & Chadha, A. (2005). “Preparation of Biodiesel from Crude Oil of Pangamia Pinnata,” Bioresource Technology, vol. 96, pp. 1425-1429.
Publisher – Google Scholar
Kovo, A. S. (2006). “Application of Fuel 42 Factorial Design for the Development and Characterization of Insecticidal Soap from Neem Oil,” Leonardo Electronic journal of practices and Technologies, pp. 29-40.
Publisher – Google Scholar
Krisnangkura, K., Sansa-ard, C., Aryusuk, K., Lilitchan, S. & Kittiratanapiboon, K. (2010). “An Empirical Approach for Predicting Kinematic Viscosities of Biodiesel Blends,” Fuel, vol. 89 pp. 2775-2780.
Publisher – Google Scholar
Neem Oil, Wikipedia, Free encyclopedia website, http://en.wikipedia.org/wiki/Neem_oil
Publisher
Neem Oil Characteristics, Wikipedia, free encyclopedia website: http://en.wikipedia.org/wikineem_oil.
Pramanik, K. (2003). “Properties and Use of Jatropha Curcas Oil and Diesel Fuel Blends in Compression Ignition Engine,” Renewable Energy, vol.28 pp. 239-248.
Publisher – Google Scholar
Ramadhas, A. S., Jayaraj, S. & Muraleedharan, C. (2005). “Biodiesel Production from High FFA Rubber Seed Oil,”Fuel, vol.84, pp. 335-340.
Publisher – Google Scholar
Sathya, T. & Manivannan, A. (2013). “Biodiesel Production from Neem Oil Using Two Step Transesterification,”International Journal of Engineering Research and Applications (IJERA), vol. 3 pp. 488-492.
Publisher
Shahid, E. M. & Jamal, Y. (2011). “Production of Biodiesel: A Technical Review,” Renewable and Sustainable Reviews, vol.9, pp. 4732-4745.
Publisher – Google Scholar
Shaine, T. K. & McCormick, R. L. (2004). “Biodiesel Handling and Use Guidelines,” U.S. Department of energy. National Renewable Energy Laboratory, pp.1—60. doi: 10.2172/15009907, Date of Publication: November 1, 2004.
Publisher
Soetaredjo, F. E., Budijanto, G. M., Prasetyo, R. I. & Indraswati, N. (2008). “Effects of Pre-Treatment Condition on the Yield and Quality of Neem Oil Obtained by Mechanical Pressing,” APRN Journal of Engineering and Applied Sciences vol.3, pp. 45-49.
Publisher – Google Scholar
Tate, R. E., Watts, K. C., Allen, C. A. W. & Wilkie, K. I. (2006). “The Densities of Three Biodiesel Fuels at Temperatures up to 300â—¦C,” Fuel, vol. 85, pp. 1004-1009.
Publisher – Google Scholar
Tate, R. E., Watts, K. C., Allen, C. A. W. & Wilkie, K. I. (2006). “The Viscosities of Three Biodiesel Fuels at Temperatures up to 300â—¦C,” Fuel, vol. 85, pp. 1010—1015.
Publisher – Google Scholar
Thangaraj, B. & Raj, S. P. (2013). “Two Stage Processes of Homogenous Catalysed Transesterification of High Free Fatty Acid Crude Oil of Rubber Seed,” International Journal of Sustainable Energy, pp.1-11. http://dx.doi.org/10.1080/14786451.2012.761220
Publisher – Google Scholar
Van Gerpen, J., Shanks, B., Pruszko, R., Clements, D. & Knoth, G. (2004). “Biodiesel Production Technology,” Lowa State University, Renewable Products Development Laboratory. USDA/NCAUR, NREL/SR-510-36244, pp. 1—105. doi: 10.2172/15008801, Date of Publication: July 1, 2004.
Publisher – Google Scholar
Wang, T., Yu, P., Wang, S. & Luo, Y. (2009). “Application of Sodium Aluminate as a Heterogeneous Base catalyst for Biodiesel Production from Soybean Oil,” Energy & Fuels, vol.23, pp. 1089-1092.
Publisher – Google Scholar