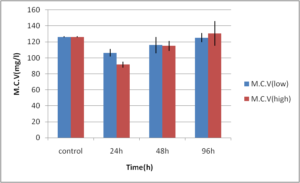
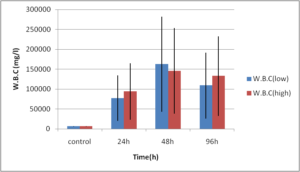
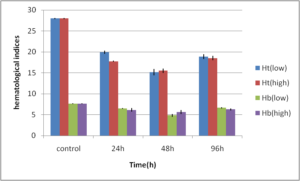
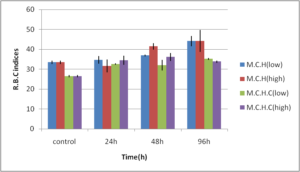
Abdel-Ghafar, F. & El-Khayat, H. (1988). Ain Shams Med. J. 39 (1988)221
Adhikari, S., Sarkar, B., Chatterjee, A., Mahapatra, C. T. & Ayyappan, S. (2004). “Effects of Cypermethrin and Carbofuran on Haematological Parameters and Prediction of their Recovery in a Freshwater Teleost, Labeo rohita(Hamilton),” Ecotoxicology and Environmental Safety 58:220-226
Publisher – Google Scholar
Affonso, E. G., Polez, V. L. P., Corre, C. F., Mazon, A. F., Araujo, M. R. R., Moraes, G. & Ratin, F. T. (2002). ‘Blood Parameters and Metabolites in the Teleosts fish Colossoma Macropomum Exposed to Sulfide or Hypoxia,’ Comparative Biochemistry and Physiology C133:375-382
Google Scholar
Allin, C. J. & Wilson, R. W. (2000). “Effects of Pre-Acclimation to Aluminium on the Physiology and Swimming Behaviour of Juvenile Rainbow Trout (Oncorhynchus Mykiss) during a Pulsed Exposure,” Aquatic Toxicology 51 (2):213-224
Publisher – Google Scholar
Ates, B., Orun, I., Talas, Z. S., Durmaz, G. & Yilmaz, I. (2008). “Effects of Sodium Selenite on Some Bio-chemical and Hematological Parameters of Rainbow Trout (Oncorhynchus Mykiss Walbaum, 1792) Exposed to Pb2+ and Cu2+,” Fish Physiology and Biochemistry 34:53-59
Publisher – Google Scholar
Bailey, S. E., Olin, T. J., Bricka, R. M. & Adrian, D. D. (1999). “A Review of Potentially Low-cost Sorbents for Heavy Metals,” Water Research 33:2469-2479
Publisher – Google Scholar
Barman, S. C. & Lal, M. M. (1994). ‘Accumulation of Heavy Metal (Zn, Cu, Cd and Pb) in Soil and Cultivated Vegetables and Weeds Grown in Industrially Polluted Fields,’ Journal of Environmental Biology 15:107-115
Beutler, E., Lichtman, M. A., Coller, B. S. & Seligsohn, U. (2001). ‘Hematology,’ sixth ed. McGraw-Hill, USA
Carvalho, C. S. & Fernandes, M. N. (2006). “Effect of Temperature on Copper Toxicity and Hematological Responses in the Neotrophical Fish, Prochilodus Scrofa at Low and High pH,” Aquaculture 251:109-117
Publisher – Google Scholar
Chowdhury, M. J., Pane, E. F. & Wood, C. M. (2004). “Physiological Effects of Dietary Cadmium Acclimation and Waterborne Cadmium Challenge in Rainbow Trout: Respiratory, Ionoregulatory, and Stress Parameters,” Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 139:163-173
Publisher – Google Scholar
Darwish, A. M., Griffin, B. R., Straus, D. L. & Mitchell, A. J. (2001). “Histological and Haematological Evaluation of Potassium Permanganate Exposure in Channel Catfish,” Journal of Aquatic Animal Health 14:134-144
Publisher – Google Scholar
Hardig, J. & Hoglund, L. B. (1983). “Seasonal and Ontogenetic Effects on Methaemoglobin and Reduced Glutathione Content in the Blood of Reared Baltic Salmon,” Comparative Biochemistry and Physiology 75:27-34
Publisher
Hedayati, A., Safahieh, A., Savari, A. & Ghofleh Marammazi, J. (2010). “Detection of Mercury Chloride Acute Toxicity in Yellowfin Sea Bream (Acanthopagrus Latus),” World Journal of Fish and Marine Sciences 2: 86-92
Publisher – Google Scholar
Hoffman, J. D., Rattner, B. A., Burton, G. A. & Cairns, J. (1995). Handbook of Ecotoxicology, Lewis Publishers, London
Publisher – Google Scholar
Hoyle, I., Shaw, B. J. & Handy, R. D. (2007). “Dietary Copper Exposure in the African Walking Catfish, Clarias Gariepinus: Transient Osmoregulatory Disturbances and Oxidative Stress,” Aquatic Toxicology 83(1):62-72
Publisher – Google Scholar
Kavitha, C., Malarvizhi, A., Kumaran, S. S. & Ramesh, M. (2010). “Toxicological Effects of Arsenate Exposure on Hematological, Biochemical and Liver Transaminases Activity in an Indian Major Carp, Catla Catla,” Food and Chemical Toxicology 48:2848-2854
Publisher – Google Scholar
Kim, S. G., Park, D. K., Jang, S. W., Lee, J. S., Kim, S. S. & Chung, M. H. (2008). “Effects of Dietary Benzo Pyrene on Growth and Hematological Parameters in Juvenile Rockfish, Sebastes Schlegeli (Hilgendorf),” Bulletin of Environmental Contamination and Toxicology 81:470-474
Publisher – Google Scholar
Kumar, S., Lata, S. & Gopal, K. (1999). “Deltamethrin Induced Physiological Changes in Freshwater CatfishHeteropneustes Fossilis,” Bulletin of Environmental Contamination and Toxicology 62: 254-258
Publisher – Google Scholar
Landis, W. G. & Yu, M. H. (2004). ‘Introduction to Environmental Toxicology,’ Crc Press. pp: 509
Google Scholar
Lee, R. G., Foerster, J., Jukens, J., Paraskevas, F., Greer, J. P. & Rodgers, G. M. (1998). ‘Wintrobe’s–Clinical Hematology,’ 10th ed. Lippincott Williams & Wilkins, New York, USA
Google Scholar
Lewis, S. D. & Lewis, W. M. (1971). ‘The Effect of Zinc and Copper on the Osmolality of Blood Serum of the Channel Catfish, Ictalurus Punctatus and Golden Shiner, Notemigonus Crysoleucas,’ Transactions of American Fisheries Society4:639-643
Li, Z. H., Velisek, J., Zlabek, V., Grabic, R., Machova, J., Kolarova, J., Li, P. & Randak, T. (2011). “Chronic Toxicity of Verapamil on Juvenile Rainbow Trout (Oncorhynchus Mykiss): Effects on Morphological Indices, Hematological Parameters and Antioxidant Responses,” Journal of Hazardous Materials 185: 870-880
Publisher – Google Scholar
Li, Z. H., Velisek, J., Zlabek, V., Grabic, R., Machova, J., Kolarova, J. & Randak, T. (2010). “Hepatic Antioxidant Status and Hematological Parameters in Rainbow Trout, Oncorhynchus Mykiss, after Chronic Exposure to Carbamazepine,”Chemico-Biological Interactions 183:98-104
Publisher – Google Scholar
Moore, M. N. (2002). “Biocomplexity: The Post-genome Challenge in Ecotoxicology,” Aquatic Toxicology 59:1-15
Publisher – Google Scholar
Nemcsok, J. & Benedeczky, I. (1990). “Effect of Sublethal Concentrations of Phenol on Some Enzyme Activities and Blood Sugar Level of Carp, (Cyprinus Carpio L.),” Environmental Monitoring and Assessment 14: 377-383
Publisher – Google Scholar
Nemcsok, J. & Boross, L. (1982). “Comparative Studies on the Sensitivity of Different Fish Species to Metal Pollution,”Acta Biologica Academiae Scientarum Hungarica 33: 23-27
Publisher – Google Scholar
Nussey, G., Van Vuren, J. H. J. & Du Preez, H. H. (1995). ‘Effects of Copper on Haematology and Osmoregulation of the Mozambique Tilapia, Oreochromis Mossambicus (Cichlidae),’ Comparative Biochemistry and Physiology 111:369-380
Panigrahi, A. K. & Misra, B. N. (1987). ‘Toxicological Effects of Mercury on a Fresh Water Fish Anabas Scandens, CUV and VAL and their Ecological Implications,’ Environmental Pollution.16:31-39
Rambhaskar, B. & Rao, K. S. (1987). “Comparative Haematology of Ten Species of Marine Fish from Visakhapatnam Coast,” Journal of Fish Biology. 30: 59-66
Publisher – Google Scholar
Remyla, S. R., Ramesh, M., Sajwan, K. S. & Kumar, K. S. (2008). “Influence of Zinc on Cadmium Induced Haematological and Biochemical Responses in a Freshwater Teleost Fish Catla Catla,” Fish Physiology and Biochemistry 34:169-174
Publisher – Google Scholar
Sancho, E., Ceron, J. J. & Ferrando, M. D. (2000). “Cholinesterase Activity and Hematological Parameters as Biomarkers of Sublethal Molinate Exposure in Anguilla Anguilla,” Ecotoxicology and Environmental Safety 46:81-86
Publisher – Google Scholar
Saravanan, M., Karthika, S., Malarvizhi, A. & Ramesh, M. (2011). “Ecotoxicological Impacts of Clofibric Acid and Diclofenac in Common Carp (Cyprinus Carpio) Fingerlings: Hematological, biochemical, Ionoregulatory and Enzymological Responses,” Journal of Hazardous Materials 195:188-194
Publisher – Google Scholar
Shah, S. L. (2002). “Behavioural Abnormalities of Cyprinion Watsoni on Exposure to Copper and Zinc,” Turkish Journal of Zoology, 26:137-140
Publisher – Google Scholar
Shah, S. L. & Altindag, A. (2005). ‘Alterations in the Immunological Parameters of Tench (Tinca Tinca L.) after Acute and Chronic Exposure to Lethal and Sublethal Mercury Treatments,’ Bulletin of Environmental Contamination and Toxicology73:911-918
Silveira-Coffignya, R., Prieto-Trujilloa, A. & Ascencio-Valle, F. (2004). “Effects of Different Stressors in Haematological Variables in Cultured Oreochromis Aureus S.,” Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 39:245-250
Publisher – Google Scholar
Sobecka, E. (2001). ‘Changes in the Iron Leveling the Organs and Tissues of Wells Catfish, Silurus Glanis L. Caused by Nickel,’ Acta. Ichthyol. Piscat. 31 (2):127-143
Soengas, J. L., Agra-Lago, M. J., Carballo, B., Andres, M. D. & Veira, J. A. R. (1996). “Effects of an Acute Exposure to Sublethal Concentrations of Cadmium on Liver Carbohydrates Metabolism of Atlantic Salmon, (Salmo Salar),” Bulletin of Environmental Contamination and Toxicology, 57(4):625-631
Publisher – Google Scholar
Stevens, M. L. (1997). Fundamentals of Clinical Hematology, WB Saunders, Philadelphia, PA
Publisher – Google Scholar
Talas, Z. S. & Gulhan, M. F. (2009). “Effects of Various Propolis Concentrations on Biochemical and Hematological Parameters of Rainbow Trout (Oncorhynchus Mykiss),” Ecotoxicology and Environmental Safety 72:1994-1998
Publisher – Google Scholar
Tellez-Banuelos, M. C., Santerre, A., Casas-Solis, J., Bravo-Cuellar, A. & Zaitseva, G. (2009). “Oxidative Stress in Macrophages from Spleen of Nile Tilapia (Oreochromis Niloticus) Exposed to Sublethal Concentration of Endosulfan,”Fish & Shellfish Immunology 27:105-111
Publisher – Google Scholar
Tseng, C.- H. (2004). “The Potential Biological Mechanisms of Arsenic-induced Diabetes Mellitus,” Toxicology and Applied Pharmacology 197:67-83
Publisher – Google Scholar
Van Vuren, J. H. J. (1986). “The Effects of Toxicants on the Hematology of Labeo Umbratus (Teleostei Cyprinidae),”Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 83:155-159
Publisher – Google Scholar
Wang, Q., Kim, D., Dionysiou, D. D., Sorial, G., A. & Timberlake, D. (2004). “Sources and Remediation for Mercury Contamination in Aquatic Systems-A Literature Review,” Environmental Pollution, 131(2):323-336
Publisher – Google Scholar
Witeska, M. (2004). ‘The Effect of Toxic Chemicals of Blood Cell Morphology in Fish,’ Fresenius Environ. Bull. 13 (12):1379-1384