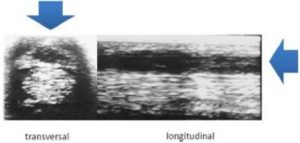
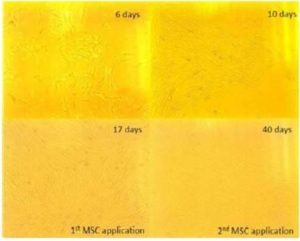
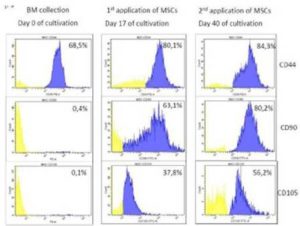
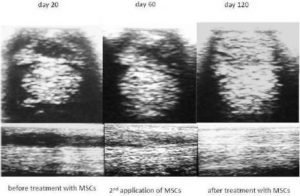
[1] Georg, R., Maria, C., Gisela, A. & Bianca, C. (2010). “Autologous Conditioned Plasma as Therapy of Tendon and Ligament Lesions in seven Horses,” Journal of Veterinary Science, 11(2) 173—175.Publisher – Google Scholar
[2] Becker, C. K., Savelberg, H. H., Buchner, H. H. & Barneveld, A. (1998). “Long-term Consequences of Experimental Desmotomy of the Accessory Ligament of the Deep Digital Flexor Tendon in Adult Horses,” American Journal of Veterinary Research, 59(3)347-51.
Publisher – Google Scholar
[3] Argüelles, D., Carmona, J. U., Climent, F., Munoz, E. & Prades, M. (2008). “Autologous Platelet Concentrates as a Treatment for Musculoskeletal Lesions in Five Horses,” Veterinary Record, 162 208-211.
Publisher – Google Scholar
[4] Smith, R. K. W., Korda, M., Blunn, G. W. & Goodship, A. E. (2003). “Isolation and Implantation of Autologous Equine Mesenchymal Stem Cells from Bone Marrow into the Superficial Digital Flexor Tendon as a Potential Novel Treatment,”Equine Veterinary Journal, 35 99-102.
Publisher – Google Scholar
[5] Bianco, P., Cao, X., Frenette, P. S., Mao, J. J., Robey, P. G., Simmons, P. J. & Wang, C. Y. (2013). “The Meaning, the Sense and the Signifikance: Translating the Science of Mesenchymal Stem Cells into Medicine,” Nature Medicine,19(1)35-42.
Publisher – Google Scholar
[6] Crovace, A., Lacitignola, L., Rossi, G. & Francioso, E. (2010). “Histological and Immunohistochemical Evaluation of Autologous Cultured Bone Marrow Mesenchymal Stem Cells and Bone Marrow Mononucleated Cells in Collagenase-induced Tendinitis of Equine Superficial Digital Flexor Tendon,” Veterinary Medicine International, 1-10.
Publisher – Google Scholar
[7] de Mattos Carvalho, A., Alves, A. L., Golim, M. A., Moroz, A., Hussni, C. A., de Oliveira, P. G. & Deffune, E. (2009). “Isolation and Immunophenotypic Characterization of Mesenchymal Stem Cells Derived from Equine Species Adipose Tissue,” Veterinary Immunology and Immunopathology,132(2-4)303-6.
Publisher – Google Scholar
[8] Richardson, L. E., Dudhia, J., Clegg, P. D. & Smith, R. (2007). “Stem Cells in Veterinary Medicine – Attempts at Regenerating Equine Tendon after Injury,” Trends in Biotechnology, 25(9)409-16.
Publisher – Google Scholar
[9] Koerner, J., Nesic, D., Romero, J. D., Brehm, W., Mainil-Varlet, P. & Grogan, S. P.(2006) “Equine Peripheral Blood-Derived Progenitors in Comparison to Bone Marrow-Derived Mesenchymal Stem Cells,” Stem Cells, 24(6)1613-9.
Publisher – Google Scholar
[10] Koch, T. G., Heerkens, T., Thomsen, P. D. & Betts, D. H. (2007). “Isolation of Mesenchymal Stem Cells from Equine Umbilical Cord Blood,” BMC Biotechnology, 726.
Publisher – Google Scholar
[11] Burk. J., Badylak, S. F., Kelly, J. & Brehm, W. (2013). “Equine Cellular Therapy-From Stall to Bench to Bedside?,”Cytometry Part A, 83(1)103-13.
Publisher – Google Scholar
[12] De Schauwer, C., Piepers, S., Van de Walle, G. R., Demeyere, K., Hoogewijs, M. K., Govaere, J. L., Braeckmans, K., Van Soom, A. & Meyer, E. (2012). “In Search for Cross-Reactivity to Immunophenotype Equine Mesenchymal Stromal Cells By Multicolor Flow Cytometry,” Cytometry Part A, 81(4)312-23.
Publisher – Google Scholar
[13] Hale, B. W., Goodrich, L. R., Frisbie, D. D., McIlwraith, C. W. & Kisiday, J. D. (2012). “Effect of Scaffold Dilution on Migration of Mesenchymal Stem Cells from Fibrin Hydrogels,” American Journal of Veterinary Research, 73(2)313-8.
Publisher – Google Scholar
[14] Kang, B. J., Ryu, H. H., Park, S. S., Kim, Y., Woo, H. M., Kim, W. H. & Kweon, O. K. (2012). “Efect of Matrigel on the Osteogenic Potential of Canine Adipose Tissue- Derived Mesenchymal Stem Cells,” The Journal of Veterinary Medical Science, 74(7)827-36.
Publisher – Google Scholar
[15] Frisbie, D. D. & Smith, R. K. W. (2010). “Clinical Update on the Use of Mesenchymal Stem Cells in Equine Orthopaedics,” Equine Veterinary Journal, 42(1)86-9.
Publisher – Google Scholar
[16] Delling, U., Lindner, K., Ribitsch, I., Jülke, H. & Brehm, W. (2012). “Comparison of Bone Marrow Aspiration at the Sternum and the Tuber Coxae in Middle-aged Horses,” Canadian Journal of Veterinary Research, 76(1) 52—56.
Publisher – Google Scholar