Introduction
Ticks are blood seeking ectoparasites adapted to very different habitats and hosts. Although the direct impact in animal health is important, the greatest significance of tick infestation is transmission of pathogens to animal and human beings. In fact, ticks are, after mosquitoes, the second most important group of vectors. For many years, tick control has been based on similar methodologies as topical acaricide administration in many parts of the world regardless of the tick species and its chronobiology. In consequence development of acaricide resistances appeared and resulted on a low efficiency of tick control strategies (Kunz and Kemp 1994). In order to be more efficient, specific control measures should be based on local tick phenology and different modes of acaricide application.
Treating domestic animals alone may not be sufficient to control ticks as the developmental stages could be maintained on wild animals. A perfect example is the tick species Hyalomma lusitanicum which is the most abundant triphasic, exophilic tick in central Spain, and a recognized vector of ruminant Mediterranean theileriosis and potential vector of several zoonotic bacterial agents (Toledo et al. 2008, 2009). Even without paying attention to its vectorial capacity or the direct effect of hyper-infestation on wild and domestic animals, the extreme abundance of host seeking stages in spring and early summer make impossible for people to enjoy open field activities in some areas.
In that context, measures directed to control free living stages of the tick species should be developed. However, the use of synthetic acaricides has a negative environmental impact (Kunz and Kemp 1994). Biopesticides are commonly used as an alternative in organic farming. Some of them, such as thymol, oxalic acid, formic acid and lactic acid, have been reported as organic alternatives for chemical acaricides (Espinosa et al., 2007; George et al., 2008). In the same sense, some authors are successfully studying the in vitro activity of many extracts of several plants and insect growth regulators against ticks (de Oliveira et al. 2011, 2012, Arnosti et al. 2010a, b, Politi et al. 2012, Ramalho et al. 2012, Calligaris et al. 2013, Roma et al. 2013) even entomopathogenic fungi in vitro (Sun et al. 2013) and in field (Valcárcel et al., 2013).
Oxalic acid, a metabolite of ethylene glycol, an ubiquitous molecule in the environment (TOXNET 2007), is one of the most common organic treatment of varroosis in honey bees worldwide (Gregorc and Planinc 2005, Gregorc and Poklukar 2003, Aliano et al. 2006, Pérez 2006) and it has been described as the virulence factor of Beauveria bassiana fungus against ticks (Kirland et al. 2005). Moreover, its effectiveness against H. lusitanicum has been established in vitro(Olmeda et al. 2008). The goal of this study was to evaluate if Oxalic acid was effective in controlling adults of H. Lusitanicum under field conditions.
Materials and Methods
Study Site
The study was conducted at a natural reserve of plant and animal species representative of the Meso-Mediterranean bioclimatic environment (Central Spain, 37º24’78”N; 42º59’101”E; HT669m). Accordingly to Köppen classification the climate is Csa (temperate with dry or hot summer) (AEMET 2011).The study site covered an area of 13,000 ha, at an altitude 500-1,266 meters above sea level. The annual average rainfall was 650 mm and temperatures ranged from -4 to43 ºC. The main trees include oaks (Quercus ilex), cork (Quercus suber), mastic (Pistacia lentiscus), eucalyptus(Eucalyptus globulus) and pines (Pinus pinea and P.pinaster). There was also a reduced agricultural activity with 265 haof organically grown olive (Olea europea) where no herbicides or insecticides were used, 1,300 ha of cereals and aromatic and native shrub species that provided food and shelter for wildlife. It was a refuge for indigenous species as Imperial eagle (Aquila adalberti), Bonelli’s eagle (Hieraaetus fasciatus), Golden eagle (Aquila chrysaetos), Peregrine hawks (Falco peregrinus), Eagle owls (Bubo bubo), Black storks (Ciconia nigra), Black vultures (Aegypius monachus); Iberian lynx (Lynx pardinus) and many other species as red fox (Vulpes vulpes), red deer (Cervus elaphus), roe deer (Capreolus capreolus), wild boar (Sus scrofa), partridge (Alectoris rufa), rabbits (Oryctolagus cuniculus), hares (Lepus europaeus) or mouse (Apodemus sylvaticus). No domestic animals are present in the study place.
Study Design
Experiments were carried out from June 2008 to October 2011. Trials were only performed from April to July because the higher host seeking ticks abundance in the area (Olmeda et al 2005, 2007; Basco-Basco et al. 2008; Cota et al 2009; 2010; Barandika et al 2011).
Eleven trials were performed using different doses of OA and two different application methods: hose (four trials, 71 replicates of 1,500 m2) and Ultra Low Volumen (ULV) device (seven trials of 4,000 m2) (Tables 1 to 2).
Table 1. Experimental Use of Oxalic Acid for Controlling Host Seeking Adults of Hyalomma Lusitanicum under Field Conditions in a Mesomediterranean Area Using Two Application Methods
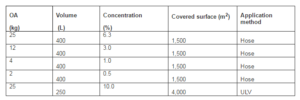
Table 2. Characteristics of the Study Area and Oxalic Acid (OA) Concentrations Applied to Control Host Seeking Ticks (Hyalomma Lusitanicum). All Experiments were Performed when Wind was Absent or Mild and the Vegetation High Ranged from 25 To 35 Cm. When Hose Application Trials, A Positive Control (5 L (Fenitrothion 25% + Cypermetrine 2.5%) /2,000 L Water) and Negative Control (Tap Water) were Used. For ULV Application Treatment Plots were Compared to Non Treated Plots
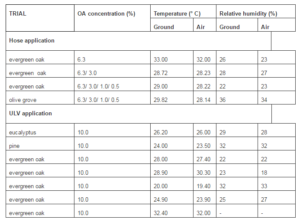
Hose Application
A latin square design, 3*3 plots to 5*5 plots, was used to minimize the animal lodging effect (tick cluster). Latin squares were divided in plots of 1,500 m2 with 10 m wide corridors. A total of 71 plots (46 evergreen oak forests and 25 olive groves) were studied in four trials covering a surface of 106,500 m2.
Three tanks were used to apply OA solutions (freshly prepared in hot tap water) (Table 1); A positive control (+C) which was 0.15% of Fito Gal® (Fenitrothion 25% + Cypermethrin 2.5%) and a negative control (-C) which included tap water were also prepared. Tanks attached to tractors were transported to field study areas. It took 45 minutes per plot to apply the 400 L of each solution at 7-8 bars of pressure.
Ultra Low Volume (ULV) Application
Seven trials were performed using an Ultra-Low Volume (ULV) vat tractor device covering a total treated surface of 28,000 m2. Other non treated plots covering 28,000 m2 were used as control. For each trial, a 410 m long path was selected divided in two plots (treated and untreated) of 200 m long x 10 m and separated by a corridor of 10 m. In treated plots a total of 250 l of 10% OA was applied for a period of 10 minutes. The speed of the tractor during the application was 1.2 Km/h.
Host Seeking Ticks
To determine tick abundance before treatments, corridors closed to the study plots were sampled using the blanket dragging technique for 30 minutes 24 hours before treatments (Toledo et al 2008, 2009). Similarly, 24 hours after treatments, ticks were sampled from each of the treated, control plots and not treated plots.
Specific flags were specifically used in different treatments (products and solutions). Samplings of host seeking ticks were carried out by five two-people. Each team represented one replicate of each treatment in order to avoid individual bias.
Complementary Data
Data of temperature and relative humidity at ground level and air, vegetation length and wind were taken on the day of treatments. All the experiments were assumed to have been performed under similar climate conditions (Table 2). No experiments were performed under strong wind or raining conditions. A visual inspection of the flora was performed during and after treatments in order to detect any side effect of the products. Berlesse technique was used to determine the effect of treatments on non-target arthropods from second to fifth hose application replications and, similarly Pitfall technique was also performed in the ULV treatments (Sabu and Shilu 2010).
Efficiency Evaluation
The Tick Abundance Rate (TAR) was estimated as the number of ticks captured during 100 minutes using the blanket dragging techniques: TAR= (tick number/ time capture)*100. As Hyalomma lusitanicum is the most abundant exofilic tick in the central Iberian Peninsula (Basco-Basco et al 2008) this report included the number of only this tick species.
To compare the efficiency of treatments, data of day +1 were used. The percentage of tick reduction comparing different treatments to negative control was calculated as: Tick reduction (%) = ((TAR in -C plots – TAR in treated plots)/ TAR in -C plots) *100.
Sign and Mann-Whitney tests were performed in order to compare differences between treatments. The significant level used was p= 0.05 to 0.01.
Results
As it was expected, non treated and negative control plots showed the highest TAR (9.4-32.6 ticks /100 minutes) after application; the lowest values were usually found in positive control plots (2.0-4.2 ticks /100 minutes); most OA concentrations also achieved significant tick reduction under field conditions (0-16.2 ticks /100 minutes) (Table 3). When oxalic acid was applied by a hose, tick reduction was variable but sometimes the efficacy was even higher than positive control.
Table 3. Reduction of Tick (Hyalomma Lusitanicum) Abundance Rate (TAR) by Hose Application of Oxalic Acid (OA) Under Field Conditions. Statistically Differences are Referred to Treated Plots Compared to Negative Control Plots
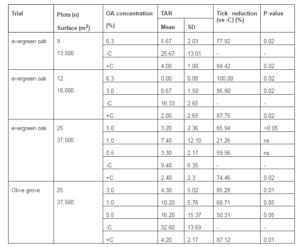
The first trial using hose application (6.3% OA) performed in an evergreen oak area reached a 77.9% of reduction of H. lusitanicum. The second treatment with the same OA concentration (6.3%) resulted in 100% reduction in TAR. Even a lower OA concentration (3.0%) resulted in a 95.9% reduction in TAR in the second trial. In the third trial, OA concentrations (3.0%, 1.0% and 0.5%) also reduced tick population (from 21.3% to 65.9%), although only the 3.0% OA had a statistically lower TAR compared to the negative control. The fourth hose trial achieved again significant tick reductions (from 50.3% to 85.3%).
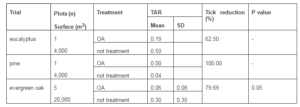
Using the ULV applicator it also reached a global significantly tick reduction of 75.86% respect the non treated plots ranging from 62.5% to 100% (Table 4).
Table 4. Reduction of Tick (Hyalomma Lusitanicum) Abundance Rate (TAR) by Ultra Low Volume Application of Oxalic Acid (10%) under Field Conditions. Statistically Differences are Referred to Treated Plots Compared to Non Treated Plots
There were no adverse effects on vegetation under visual inspection with the exception of just one application of OA 3.0% performed by hose on evergreen oak forest. In this occasion Lavandula flowers were slightly affected by treatment (flowers seemed to wither) but recovered in a few days. The number of non target arthropods in treated and not treated plots were not significantly different (unpublished data).
Discussion
When there is a supper population of ticks in an area, different strategies must be applied for tick control, especially in large areas where the management of animals is difficult as it happens in domestic extensive systems or in game reserves. In these situations, targeting the treatments to host seeking ticks could be very helpful to design a suitable integrated control that allows the reduction of direct/indirect tick effects, transmitted pathogens, and human labour lost, drugs residues in animal products and others. From this point of view, these results seem to indicate that oxalic acid is an alternative useful for tick control.
Oxalic acid is a metabolite of ethylene glycol ubiquitous in the natural medium, both in the smoke of snuff, vegetables and soil or atmosphere (TOXNET 2007). It also forms part, as the potassium salt or calcium, plants and mammals (urine, bones and others). It is one of the most common organic treatments in the varroosis of bees and it seems to becompatible with organic farming and would be relatively safe for other arthropods. Under these conditions there were not severe side effects on plants and non targeted arthropods but further specific studies should be done to confirm that.
Both application methods were effective in the control of host seeking ticks. The hose treatment application needs just a worker that spent about 45 minutes to treat a 1,500 m2 plot so the labor cost of application is affordable in small areas. Using this system, OA always reduced the level of H. lusitanicum. Although administering the higher dose (OA 6.3%) the best performances were obtained, the difficulty of this dose preparation and the product consumption recommend us to use fewer active substances to optimize the results. In consequence, the intermediate OA concentration (3.0%) always showed significant reductions and quite similar to 6.3%, avoiding preparation troubles and reducing product consumption.
The ULV application was also effective against host seeking ticks allowing to treat large surfaces very quickly reducing time consumption (one worker can treat a 4,000 m2 plot in just ten minutes) and so the human cost. Once ULV applicator is disposable it seems to be faster and cheaper than hose system and it spends less product (25.00 kg and 66.67 kg per 4,000 m2; ULV and hose, respectively). As it is usual when a treatment is performed on field it is important to note that personal protection measures should be taken and no animals or human presence must be allowed when product is applied until five-ten minutes post ULV application.
In conclusion, application of oxalic acid by hose or ULV device methods can be used to control of host seeking adults ofHyalomma lusitanicum tick. So it could be a useful and realistic alternative to traditional acaricides under field conditions, especially when resistance to commercial drugs is developed.
Acknowledgements
Authors are especially grateful to his Grace the Duke of Westminster for his support during all the studies.
Project has been financed by the Villamagna SA and the project CCG10-UCM/AMB-4936, INCRECYT (European Social Funds). The contract of Dr. Pérez was financed by subprogram INNCORPORA from the Ministerio de Economía y Competitividad, Spanish Government.
References
AEMET (Agencia Estatal de Meteorología). (2011). Iberian Climate Atlas. Ministerio de Medio Ambiente Y Medio Rural Y Marino, Gobierno de Espa-a. Madrid, Spain.
Publisher
Aliano, N. P., Ellis, M. D. & Siegfried, B. D. (2006). “Acute Contact Toxicity of Oxalic Acid to Varroa Destructor (Acari: Varroidae) and Their Apis Mellifera (Hymenoptera: Apidae) Hosts in Laboratory Bioassays,” Journal of Economic Entomology, 99(5): 1579-1982.
Publisher – Google Scholar
Arnosti, A., Brienza, P. D., Furquim, K. C. S., Chierice, G. O., Bechara, G. H., Calligaris, I. B. & Camargo-Mathias, M. I. (2010). “Effects of Ricinoleic Acid Esters from Castor Oil of Ricinus Communis on the Vitellogenesis of Rhipicephalus Sanguineus (Latreille, 1806) (Acari: Ixodidae) Ticks,” Experimental Parasitology, 127(2): 575-580.
Publisher – Google Scholar
Arnosti, A., Brienza, P. D., Furquim, K. C. S., Chierice, G. O., Neto, S. C., Bechara, G. H., Sampieri, B. R. & Camargo-Mathias, M. I. (2010). “Effects of Ricinus Communis Oil Esters on Salivary Glands of Rhipicephalus Sanguineus (Latreille, 1806) (Acari: Ixodidae),” Experimental Parasitology, 127(2): 569-574.
Publisher – Google Scholar
Barandika, J. F., Olmeda, A. S., Casado-Nistal, M. A., Hurtado, A., Juste, R. A., Valcárcel, F., Anda, P. & García-Pérez, A. L. (2011). “Differences in Questing Tick Species Distribution between Atlantic and Continental Climate Regions in Spain,” Journal of Medical Entomology, 48(1): 13-19.
Publisher – Google Scholar
Basco-Basco, P. I., Carballedo Ãlvaro, A. D., Cota Guajardo, S. C., Olmeda García, A. S. & Valcárcel, F. (2008). “Estudio de Control Biológico de Garrapatas en la Finca “La Garganta”,” Revista Complutense De Ciencias Veterinarias2(2): 73-84.
Publisher – Google Scholar
Calligaris, I. B., De Oliveira, P. R., Roma, G. C., Bechara, G. H. & Camargo-Mathias, M. I. (2013). “Action of the Insect Growth Regulator Fluazuron, the Active Ingredient of the Acaricide Acatak(®), In Rhipicephalus Sanguineus Nymphs (Latreille, 1806) (Acari: Ixodidae),” Microscopy Research and Technique 76(11): 1177-1185.
Publisher – Google Scholar
Cota-Guajardo, S., Pérez-Sánchez, J. L., Valcárcel, F., Basco-Basco, P. I., Carballedo, A. D. & Olmeda, A. S. (2009).Monitoring the Effect of Oxalic Acid against Tick Population and Non Target Arthropods in a Meso-mediterranean Environment. In Proceedings, 57th Annual Meeting of the Entomological Society of America. 11 to 16 of December 2009. Virtual Poster.
Publisher
Cota-Guajardo, S., Pérez-Sánchez, J. L., Valcárcel, F., Basco-Basco, P. I., Carballedo, A. D. & Olmeda, A. S. (2010). ‘Hyalomma Lusitanicum Phenology in a Mesomediterranean Environment of Central Spain,’ In Proceedings, International Conference EDEN 2010: Emerging Vector-Borne Diseases in a Changing European Environment. 10 to 12 of May of 2010, Montpellier, France.
De Oliveira, P. R., Calligaris, I. B., Roma, G. C., Bechara, G. H. & Camargo-Mathias, M. I. (2012). “Fluazuron-Induced Morphophysiological Changes in the Cuticle Formation and Midgut of Rhipicephalus Sanguineus Latreille, 1806 (Acari: Ixodidae) Nymphs,” Parasitology Research 112(1): 45-58.
Publisher – Google Scholar
De Oliveira, P. R., Calligaris, I. B., Roma, G. C., Bechara, G. H., Pizano, M. A. & Camargo Mathias, M. I. (2012). “Potential of the Insect Growth Regulator, Fluazuron, in the Control of Rhipicephalus Sanguineus Nymphs (Latreille, 1806) (Acari: Ixodidae): Determination of the LD95 and LD50,” Experimental Parasitology, 131(1): 35-39.
Publisher – Google Scholar
Espinosa-Monta-o, L. G. & Guzman-Novoa, E. (2007). “Eficacia de Dos Acaricidas Naturales, Ãcido Fórmico Y Timol, Para El Control Del Ãcaro Varroa Destructor de las Abejas (Apis Mellifera L.) En Villa Guerrero, Estado de México, México,” Vet. Méx., 38 (1).
Publisher – Google Scholar
Goerge, D. R., Guy, J. H., Arkle, S., Harrington, D., De Luna, C., Okello E. J., Shiel, R. S., Port, G. & Sparagano, O. A. E. (2008). “Use of Plant-Derived Products to Control Arthropods of Veterinary Importance: A Review,” Animal Biodiversity and Emerging Disseases: Annals of the New York Academy of Sciences 1149: 23-26.
Publisher – Google Scholar
Gregorc, A. & Planinc, I. (2002). “The Control of Varroa Destructor Using Oxalic Acid,” The Veterinary Journal, 163(3): 306-310.
Publisher – Google Scholar
Gregorc, A. & Poklukar, J. (2003). “Rotenone and Oxalic Acid as Alternative Acaricidial Treatments for Varroa Destructor in Honeybee Colonies,” Veterinary Parasitology, 111: 351-360.
Publisher – Google Scholar
Kirkland, B. H, Eisa, A. & Keyhania, N. O. (2005). “Oxalic Acid as a Fungal Acaracidal Virulence Factor,” Journal of Medical Entomology, 42 (3): 346-351.
Publisher – Google Scholar
Kunz, S. E. & Kemp, D. H. (1994). “Insecticides and Acaricides: Resistance and Environmental Impact,” Revue Scientifique et Technique (International Office of Epizootics), 13(4): 1249-1286.
Publisher – Google Scholar
Olmeda, A. S., Casado Nistal, M. A., Toledo, A., Meana, A. & Valcárcel, F. (2005). ‘Global Study on the Presence and Seasonal Activity of Ticks in Madrid and Castilla-La Mancha,’ In Proceedings, IX Congresso Ibérico De Parasitología. 25 to 28 of October 2005. Coimbra, Portugal.
Olmeda, A. S., Casado Nistal, M. A., Toledo, A. & Valcárcel, F. (2007). ‘Patrón de Distribución, Dinámica Estacional Y Rango de Hospedadores de las Garrapatas de la Zona Centro de la Península Ibérica (CAM, CCLM),’ In Proceedings, XII Congreso Ibérico de Parasitología.15 to 20 of July 2007. Madrid, Spain.
Olmeda, A. S., Pérez, J. L., Martín Hernández, R., Torrente, M. & Valcárcel, F. (2008). “Toxicity of Oxalic Acid Against Adult Hyalomma Lusitanicum Ticks (Ixodida: Ixodidae) In Laboratory Conditions: LD50,” Journal of Medical Entomology,45(4). 715-719.
Publisher – Google Scholar
Pérez, J. L. (2006). ‘Control Integral de la Varoosis Mediante Terapia Farmacológica Con Productos Compatibles Con La Apicultura Ecológica,’ Ph.D Dissertation. Universidad Complutense de Madrid. Madrid.
Politi, F. A. S., Figueira, G. M., Araújo, A. M., Sampieri, B. R., Mathias, M. I., Szabó, M. P., Bechara, G. H., Dos Santos, L. C., Vilegas, W. & Pietro, R. C. (2012). “Acaricidal Activity of Ethanolic Extract from Aerial Parts of Tagetes Patula L. (Asteraceae) Against Larvae and Engorged Adult Females of Rhipicephalus Sanguineus (Latreille, 1806),”Parasites & Vectors, 5:295.
Publisher – Google Scholar
Ramalho Vendramini, M. C., Camargo-Mathias, M. I., De Faria, A. U., Bechara, G. H., De Oliveira, P. R. & Roma, G. C. (2012). “Cytotoxic Effects of Andiroba Oil (Carapa Guianensis) In Reproductive System of Rhipicephalus Sanguineus (Latreille, 1806) (Acari: Ixodidae) Semi-Engorged Females,” Parasitology Research, 111:1885-1894.
Publisher – Google Scholar
Roma, G. C., Vendramini, M. C., Camargo-Mathias, M. I., Nunes, P. H., De Faria, A. U. & Bechara, G. H. (2013). “Action of Andiroba Oil and Permethrin on the Central Nervous and Reproductive Systems of Rhipicephalus Sanguineus (Latreille, 1806) (Acari: Ixodidae) Ticks Females. A Confocal Study,” Research in Veterinary Sciences, 95(2): 529-36.
Publisher – Google Scholar
Sabu, T. K. & Shiju, R. T. (2010). “Efficacy of Pitfall Trapping, Winkler and Berlesse Extraction Methods for Measuring Ground-Dwelling Arthropods in Moist-Deciduous Forests in the Western Ghats,” Journal of Insect Science, 10: 98.
Publisher – Google Scholar
Sun, M., Ren, Q., Guan, G., Li, Y., Han, X., Ma, C., Yin, H. & Luo, J. (2013). “Effectiveness of Beauveria Bassiana Sensu Lato Strains for Biological Control against Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae) In China,”Parasitology International 62(5): 412-415.
Publisher – Google Scholar
Toledo, A., Olmeda, A. S., Escudero, R., Jado, I., Valcárcel, F., Casado-Nistal, M. A., Rodríguez-Vargas, M., Gil, H. & Anda, P. (2009). “Tick—Borne Zoonotic Bacteria in Ticks Collected from Central Spain,” American Journal of Tropical Medicine and Hygiene, 1(81): 67-74.
Publisher – Google Scholar
Toledo, A., Jado, I., Olmeda, A. S., Casado-Nistal, M. A., Gil, H., Escudero, R. & Anda, P. (2009). “Detection of Coxiella Burnetii in Ticks Collected from Central Spain,” Vector-Borne and Zoonotic Diseases, 6(8): 829-835.
Publisher – Google Scholar
TOXNET (2007). Oxalic Acid. Toxicology Data Network. National Library of Medicine, United States. http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search. Date: 06/04/07
Valcárcel, F., Pérez Sánchez, J. L., Tercero Jaime, J. M., Cutuli, M. T. & Olmeda, A. S. (2013). ‘Control de la Parasitación Por Garrapatas en Conejos Silvestres Mediante Aplicación en Conejeras de Hongos Entomopatógenos,’Proceedings of the XVIII Congress of the Spanish Society of Parasitology, Gran Canaria, Islas Canarias, Spain.







