Introduction
Surgical anastmosis of various parts of intestine is most commonly performed in animals especially in dogs and cats to relive out the irresolvable intestinal obstruction or de-vitalization (Tobias and Ayres, 2006; Gregory, 2003; Rasmussen, 2002). Jejunum is the longest part of small intestine that increases its possible chance to the surgical intervention at some specific location (Dilawar et al., 2011).
Surgical wound infections are frequent complications following gastrointestinal tract surgery. Infections related to surgical wound consequence in the administration of additional antimicrobial agents. It is extensively approved that appropriate antimicrobial prophylaxis is favorable in gastrointestinal surgery that has high risk to intestinal flora (Dellinger et al.,1994).
Prior 1970, the intestinal anastomotic resections were fraught with post-operative infectious complications which occurred in more than 30-50% of all the surgical approaches to the intestine. Due to recognition of the importance of appropriate prophylactic antimicrobial therapy directed at both aerobic and anaerobic species of bacteria, surgeons have been able to perform single-stage intestinal resections with primary anastomosis routinely. The current century brought the revolution in gastrointestinal surgery, resulting in significant decrease in infectious complications, in animals including dogs with the reduced postoperative complications due to the antibiotic prophylaxis, improved surgical techniques, new anesthesia drugs and protocols as well as both pre & post-surgical management (Nichols et al., 2005). The prophylactic use of antimicrobial agents is the current standard of care prior to gastrointestinal surgery (Basany et al., 2005).
Recently, pre-operative prophylactic antibiotics became a foundation of therapy, the accurate and judicious administration of antibiotics have become performance measures for the excellence perfection and safe surgical procedures worldwide. As it is obvious from the published literature, to minimize the chances of postoperative infections for the gastrointestinal surgical procedures, the prophylactic use of different antibiotics has become the current standard of care. Metronidazole and ceftriaxone sodium are most commonly administered prophylactic agents for gastrointestinal anastomosis and considered as best choice as compared to other options. Keeping in view the highlighted facts, the purpose of present study was to evaluate the comparative effect of most commonly used prophyactic metronidazole and ceftriaxone sodium on the tensile strength of the jejunal anastmotic segment of dogs.
Material and Methods
The study comprised of twenty healthy stray dogs that were randomly divided into four groups, with five animals in each group. The studying animals included twelve males and eight females weighing between 14 to 18 kilograms. Each studied group included three male and two female stray dogs. These experimental dogs were identified by collar tags. The dogs of Group-I were kept as control and operated for jejunal anastomosis without administering any prophylactic antibiotic regime. The animals of Group-II were only given prophylactic metronidazole at the dose rate of 50 mg/Kg body weight intravenously, two hours before surgery. While the Group-III dogs were administered intravenously with ceftriaxone-sodium alone at the dose rate of 30 mg/Kg, two hours before surgical intervention. The intravenous administration of metronidazole and ceftriaxone-sodium were given in combination to animals of Group-IV at the dose rate of 50 mg/Kg and 30 mg/Kg body weight respectively, two hours prior to surgery (Hinchey et al., 1983; Woodfield et al., 2003).
Clinical Examination
Before experimentation, acclimatizing period of fourteen days was provided to all the dogs, so that they get used to the new environment to minimize stress factors. During this acclimatization period, the dogs were subjected to thorough physical and clinical examination for the evaluation of their health status. Hematological parameters including hemoglobin, hematocrit values, total erythrocyte and leukocyte count were determined by following the methods as described by Jain (1986). Experimental dogs were kept in-door in separate clean stainless steel cages for two weeks pre-operatively to rule out the possibility of any latent or nosocomial infection. Physiological parameters like body temperature, pulse and respiration rates were also recorded daily for one week preoperatively to obtain the baseline data and two weeks following surgical intervention. The experimental dogs were fed on bread and milk. Fresh water was available ad libitum except three hours before surgical intervention and six hours post-operatively.
Surgical Procedure
Before the administration of thiopentone sodium (general anesthetic agent), each dog from all four groups was pre-medicated by administering atropine-sulphate @ 0.045 mg/ Kg body weight through subcutaneous (S/C) route, half an hour prior to surgical intervention to minimize secretions of the salivary glands and respiratory tract (Plumb, 2008). Thiopentone sodium was administered by the slow vein puncture of cephalic vein, at a dose rate of 25 mg/ Kg body weight. Then laprotomy procedure was performed by following adequate pre-operative measures. Each dog was positioned as 300 tilted to the horizon in the dorsal recumbency and umbilicus was considered as surgical landmark. Ventral midline incision of about 12 cm length was made through the skin and subcutaneous tissue with the help of scalpel blade. Then peritoneal cavity was opened carefully by a stab incision and the falciform ligament was resected with scissors. This permitted uniform peritoneal contact at the closure, and minimized any risk of wound dehiscence (Tavakoli et al., 2007). After exposing the abdominal viscera; a part of the jejunum was selected and packed off for resection. Common jejunal and mesenteric arteries supplying to the selected part were isolated and ligated. The jejunum was transected with a scalpel between the crushing and non-crushing forceps along the edges of the crushing forceps. The resected section of the jejunum, with the two crushing forceps, was removed. The ansatomotic site of jejunum was then examined critically for the patency and anastomotic leaks. Suturing in three layers was accomplished for the closure of the abdomen (Weisman et al., 1999; Fossum, 2007).
After the surgical intervention each dog was then observed for recovery and placed back in the dog ward after recovery. Water was offered ad libitum followed by the milk on the postoperative day 2 and a soft diet on day 3 postoperatively. Body temperature, pulse and respiration readings were recorded twice a day for the fourteen days after operation. Each animal was given complete antiseptic dressing once daily. After 14 days, all the dogs were euthanized to find out the effect of treatments on the tensile strength.
Tensile Strength Measurement
The tensile strength (TS) of the anastomotic site was recorded at day 14th post-operatively with a device Schopper’s tensile strength tester No. 114-SC type (Yasuda Seiki Seisakusho, Japan). The instrument is specially designed for measuring the tensile strength of vinyl, rubber, leather, copper-wire, fabric and cord etc. This tester is designed and manufactured in accordance with Schopper’s mechanism on the basis of principle of balance for measuring tensile strength. For this purpose, the 6 cm intestinal segments containing jejunal anastomotic site were cut 14 days after the operation by euthanizing the all dogs. Following washing of the specimen with normal saline, three 1 x 2 cm strips containing the anastomotic site in the center and three same sized strips from the un-operated part of the jejunum (as control) were cut. Each piece was clumped in the upper and lower chucks of the Schopper’s Tensile Strength Tester leaving 1 cm2 area on the either side of the healing line and subjected to the instrument for the measurements. Tensile strength was noted as the force units (grams) indicated by the pointer on the holding scale of the tensile tester when the rupture occurred. Similar strips from the healthy (control strips) intestinal segment of all dogs were also evaluated for their tensile strength. The percent gain in the tensile strength (using means of 3) was calculated by the formula (Booth, 1968).
Gain in the tensile strength = Mean TS of strips with anastomotic site X 100
Mean TS of control strips
Results
The average gain in the tensile strength of the Group I animals was 26.50 percent. For this group, the results for gain in tensile strength were ranging between 22-31 percent. The lowest gain in tensile strength, in this group, was 22.73 percent. While the highest gain in tensile strength 30.95 percent was recorded in this group. The dogs that were prophylactically administered with metronidazole (Group II) revealed 39.70 percent average gain in the tensile strength. The percentage gain in tensile strength of this group was found to be significantly higher than Group I. The gain in tensile strength of metronidazole treated group was ranging between 37-42 percent. The highest recorded gain in tensile strength was 41.67 percent, while 37.50 percent was the lowest recorded gain in tensile strength. The group of dogs that were prophylactically administered with only ceftriaxone sodium (Group III), the highest average gain in tensile strength 44.83 percent was recorded. The average gain in tensile strength of this group 43.6 percent was significantly higher from Group I and Group II. The gain in tensile strength of this group was ranging between 41-45 percent, with highest gain in tensile strength recorded as 44.83 percent. While 41.67 was the lowest gain in tensile strength of this group. The average gain in tensile strength recorded, for the animals that were prophylactically administered with combination of ceftriaxone sodium and metronidazole (Group IV), was 47.50 percent which is significantly higher than the dogs of Group I, Group II and Group III. The gain in tensile strength of this group was ranging between 44-50 percent with the highest value of gain in tensile strength recorded as 50.0 percent. However, the lowest gain in tensile strength of this group was 44.0 percent.
Table I: Pre-Operative Prophylactic Effect of Treatment Groups on Tensile Strength of Anastomotic Portion of Jejunum
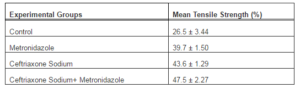
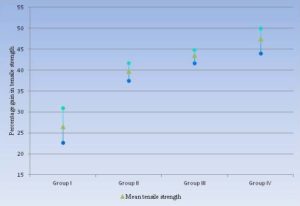
Figure 1: Graphical Representation for the Gain in Tensile Strength of Studied Groups
Statistical Analysis
The analysis of variance (ANOVA) statistical test was applied to the data obtained from the findings of mean tensile strength of each group and results expressed that among all the four studied groups, a highly significant difference was observed as probability is less than 0.01 (P < 0.01). The highest percentage gain in tensile strength of jejunum 47.5 ± 2.27 percent was observed in animals that were treated with combination of Ceftriaxone Sodium and Metronidazole (Group IV). While the lowest percentage gain in tensile strength 26.5 ± 3.44 was recorded in animals of control group (Group I).
Discussion
Restoration of normal tissue strength is among the most accepted and reliable indices of wound healing (Van Winkle, 1969; Athar et al., 1996). In case of intestinal anastomosis, tensile strength is not a function of the length of the incision or of the thickness of the tissue rather, it is determined in term of load applied per unit of cross section area. In the present study, increase in the tensile strength of the anastomosed jejunal segment was measured on day 14 postsurgical intervention following the techniques of Athar et al., (1996). Jejunal anastomotic segments of the dogs that were administered prophylactically with single dose of intravenous ceftriaxone sodium alone (Group III) and in combination with metronidazole (Group IV) had statistically highly significant increments in tensile strength (Table I) as compared to the remaining two regimens i.e. prophylactically single dose intravenous administration of metronidazole alone (Group II) and control group that was not given any prophylactic antibiotic (Group I). Moreover, the jejunal anastomotic segments of the dogs that were administered pre-operatively with single intravenous dose of metronidazole (Group II) also had statistically significant increase in tensile strength (Table I) as compared to control group (Group I). The least gain in tensile strength was observed in control group and it might be related to the presence of infective organisms at the anastomotic site.
In all three treatment groups (Group II, GroupIII and Group IV), the gain in the tensile strength seems to be due to reduction in the colonization of normal and pathogenic micro-organisms, which on getting chance might contaminate the anastomotic wound and ultimately causing post-operative infections. These results concur with those reported by Atharet al., (1996), Hayashi and Wilson (2009), Wang et al., (2003), and Woodfield et al., (2003). Statistically lesser gain in tensile strength recorded in the control animals. It was observed that during study, the animals of this group had suffered from fever for three to four days that’s why the gain in the tensile strength was lower than the groups having single dose of intravenous antibiotic prophylaxis. These findings are also inline with those of Athar et al., Cai (1992) , Leaper (1994), Lindhagen et al., (1981), Mittermayer et al., (1984) Ono et al., (1990) Parker et al., (1985) Playforth et al., (1988) and Takesue et al., (2000). As for the choice of antimicrobial agents, it is general consensus worldwide that administration of prophylactic antibiotics should be intravenous as single dose preferably 1 hour to 30 minutes before surgery. In our study it is thus not surprising that ceftriaxone sodium in combination with metronidazole has more prophylactic efficacy. This high prophylactic efficacy might be due to specific pharmacokinet profile of these antimicrobials which ensures the bactericidal actions against aerobic and anaerobic pathogenic organisms. Similar results for prophylactic efficacy of ceftriaxone sodium in combination with metronidazole also discussed by Athar et al., (1996), Gravante and Caruso(2009), Lalla (2009), Rau et al., (2000) and Ross et al., (2009).
Acknowledgement
The authors are grateful to Prof. Dr. Ghulam Muhammad, who shared the valuable information. During this study, their kind and encouraging attitude is highly appreciateable.
References
Athar, M., Chaudhry, N. I., Shakoor, A. & Khan, M. A. (1996). “Studies on End-to-End Colonic Anastomosis in the Dog: A Comparison of Techniques,” Acta Veterinaria Hungarica, 44, 349-356.
Publisher – Google Scholar
Basany, E. E., Garcia, J. L. S., Cano, M. L., Trujillo, R. L., Ferrer, M. M., Gil, L. A., Vilches, L. A. & Carrasco, M. A. (2005). “Prospective, Randomised Study on Antibiotic Prophylaxis in Colorectal Surgery. Is It Really Necessary to Use Oral Antibiotics?,” International Journal of Colorectal Disease, 20 (10) 542-546.
Publisher – Google Scholar
Booth, J. E. (1968). Principles of Textile Testing: An Introduction to Physical Methods of Testing Textile Fibres, Yarns and Fabrics, Kingsway, London, UK.
Publisher – Google Scholar
Cai, C. J. (1992). “Clinical Study of Prophylactic Use of Gentamicin and Metronidazole in the Surgery of Colorectal Carcinoma,” Zhonghua Wai Ke Za Zhi, 30 (4) 237-240.
Publisher – Google Scholar
Dellinger, E. P., Gross, P. A., Barett, I. L., Krause, P. J., Martone, W. J., Mcgowan, J. E., Sweet, K. L. & Wenzel, R. P. (1994). “Quality Standards for Antimicrobial Prophylaxis in Surgical Procedures,” Clinical Infectious Diseases, 18, 422-427.
Publisher – Google Scholar
Dilawar, M. S., Khan, M. A., Zain-Ul-Abidin, Azeem, S., Majeed, K. A., Shahbaz, A. & Khan, A. R. (2011). “Effect of Variable Degrees of Jejunal Resection upon Different Clinico-Biochemical Parameters in Dogs,” Korean Journal of Veterinary Research, 51 (4) 309-311.
Publisher – Google Scholar
Fossum, T. W. (2007). Small Animal Surgery, Mosby, London, UK.
Publisher – Google Scholar
Gravante, G. & Caruso, R. (2009). “Mechanical Bowel Preparation for Elective Colorectal Surgery: Is It Enough?,”Journal of Gastrointestinal Surgery, 13 (10) 1392-1394.
Publisher – Google Scholar
Gregory, S. (2003). ‘Gastric Outflow Tract Disease,’ Royal Vet College, London, UK.
Hayashi, M. S. & Wilson, S. E. (2009). “Is There a Current Role for Preoperative Non-Absorbable Oral Antimicrobial Agents for Prophylaxis of Infection after Colorectal Surgery?,” Surgical Infections, 10 (3) 285-288.
Publisher – Google Scholar
Hinchey, E. J., Richard, O. K. & Prentis, J. (1983). “Metronidazole as a Prophylactic Agent in Wound Infection after Colon Surgery,” Surgery, 93, 197-200.
Publisher – Google Scholar
Jain, N. C. (1986). ‘Schalms Veterinary Haematology,’ Philadelphia, USA.
Lalla, F. D. (2009). “Antimicrobial Prophylaxis in Colorectal Surgery: Focus on Ertapenem,” Therapeutics and Clinical Risk Management, 5, 829-839.
Publisher – Google Scholar
Leaper, D. J. (1994). “Prophylactic and Therapeutic Role of Antibiotics in Wound Care,” The American Journal of Surgery, 167, 155- 195.
Publisher – Google Scholar
Lindhagen, J., Hadziomerovic, A., Nordlung, S. & Zbornik, J. (1981). “Comparison of Systemic Prophylaxis with Metronidazole-Fosfomycin and Metronidazole-Cephalothin in Elective Colorectal Surgery,” Acta Chirurgica Scandinavica,147 (4) 277-283.
Publisher – Google Scholar
Mittermayer, H., Gross, C. & Brucke, P. (1984). “Single Dose Cefuroxime/ Metronidazole versus Metronidazole Alone in Elective Colorectal Surgery,” The American Surgeon, 50 (8) 418-423.
Publisher – Google Scholar
Nichols, R. L., Choe, E. U. & Weldon, C. B. (2005). “Mechanical and Antibacterial Bowel Preparation in Colon and Rectal Surgery,” Chemotherapy, 51 (10) 115-121.
Publisher – Google Scholar
Ono, S., Kato, S., Tanaka, T., Ishibiki, K., Kodaira, S. & Abe, O. (1990). “Preoperative Oral Antimicrobial Bowel Preparations in Elective Colorectal Surgery,” Nippon Geka Gakkai Zasshi, 91 (8) 972-979.
Publisher – Google Scholar
Parker, M. C., Ashby, E. C., Nicholls, M. W., Dowding, C. H. & Brookes, J. C. (1985). “Povidone-Iodine Bowel Irrigation before Resection of Colorectal Carcinoma,” Annals of the Royal College of Surgeons of England, 67 (4) 227-228.
Publisher – Google Scholar
Playforth, M. J., Smith, G. M. R., Evans, M. & Pollock, A. V. (1988). “Antimicrobial Bowel Preparation; Oral, Parenteral or Both,” Diseases of the Colon & Rectum, 31, 90-93.
Publisher – Google Scholar
Plumb, D. C. (2008). Veterinary Drug Handbook, Wiley-Blackwell Publishing Co, USA.
Publisher – Google Scholar
Rasmussen, L. (2002). Textbook of Small Animal Surgery, WB Saunders Co, Philadelphia, USA.
Publisher
Rau, H. G., Mittelkotter, U., Zimmermann, A., Lachmann, A., Kohler, L. & Kullmann, K. H. (2000). “Perioperative Infection Prophylaxis and Risk Factor Impact in Colon Surgery,” Chemotherapy, 46, 353-363.
Publisher – Google Scholar
Ross, D., Dijksman, L. M., Sondermeijer, B. M., Straaten, H. M. O., Dewit, L. T. & Gerhards, M. F. (2009). “Perioperative Selective Decontamination of Digestive Tract (SDD) in Elective Colorectal Surgery,” Journal of Gastrointestinal Surgery, 13 (10) 1839-1844.
Publisher – Google Scholar
Takesue, Y., Yokoyama, I., Akagi, O. S., Ohge, H., Murakami, Y., Sakashita, Y., Miyamoto, K., Vemura, K., Itaha, H. & Matsuura, Y. (2000). “A Brief Course of Colon Preparation with Oral Antibiotics,” Surgery Today, 30, 112-126.
Publisher – Google Scholar
Tavakoli, A., Bakhtiari, J. & Khalaj, A. (2007). “Clinical Evaluation of Two Suture Pattern Techniques in Laparoscopic Gastrojejunostomy in Dog,” Iranian Journal of Veterinary Surgery, 2 (2) 39-45.
Publisher – Google Scholar
Tobias, K. M. & Ayres, R. (2006). “Key Gastrointestinal Surgeries: Intestinal Anastomosis,” Veterinary Medicine, 1-6.
Publisher – Google Scholar
Van-Winkle, W. (1969). ‘Tensile Strength of Wound and Factors That Influence It,’ Surg Gynecol Obstet, 129, 819-842.
Google Scholar
Wang, X. C., Gui, C. Q. & Zheng, Q. S. (2003). “Combined Therapy of Allantoin, Metronidazole, Dexamethasone on the Prevention of Intra-Abdominal Adhesion in Dogs and Its Quantitative Analysis,” World Journal of Gastroenterology, 9 (3) 568-571.
Publisher – Google Scholar
Weisman, D. L., Smeak, D. D., Birchard, S. J. & Zweigart, S. L. (1999). “Comparison of a Continuous Suture Pattern with a Simple Interrupted Pattern for Enteric Closure in Dogs and Cats: 83 Cases (1991-1997),” Journal of the American Veterinary Medical Association, 214 (10) 1507-1510.
Publisher – Google Scholar
Woodfield, J. C., Van Rij, A. M., Pettigrew, R. A., Van Der Linden, A. J., Solomon, C. & Bolt, D. (2003). “A Comparison of the Prophylactic Efficacy of Ceftriaxone and Cefotaxime in Abdominal Surgery,” The American Journal of Surgery, 185 (1) 45-49.
Publisher – Google Scholar





