Introduction
The increasing specialization of artificial insemination (AI) programs in sheep production demands that sperm collection and processing centers (SCPC) become adjusted to the requirements of regulatory agencies. The World Organization for Animal Health (OIE, 2009) recommends the inclusion of antibiotics in extenders used in cryopreservation of sperm, in order to control bacterial contamination. In Brazil, these requirements are clearly defined for SCPCs that process sperm from bulls and water buffalos, but no specific regulation is established for ram sperm.
The inclusion of antibiotics in extenders is recommended for the control of several microorganisms that can be present in sperm (Bielansky, 2007; Thibier and Guerin 2000). Although opportunistic contaminants generally do not incur an important health risk for the inseminated females, they can affect sperm quality (Yániz et al., 2010). Antibiotic associations capable of controlling potentially pathogenic agents have been determined for bull sperm, having no negative effects on sperm quality (Lorton and Sulivan, 1988; Shin et al., 1988).For ram sperm, the determination of antibiotic associations mainly focused on cooled sperm (Yániz et al., 2010), and demonstrating different levels of antibiotic resistance during preservation of cooled sperm (Yániz et al., 2010; Azwi, 2012). In cryopreserved sperm, agents such as Brucella ovis and Actinobacilus seminis can be controlled by the inclusion of gentamicin or the association of penicillin and streptomycin in extenders (Moustacas et al., 2010). One might consider that bacterial populations and their levels of resistance can vary, and that the efficiency can be affected by extender components (Azwi, 2012). Additionally, the direct effect of antibiotics on sperm viability should also be considered (Lorton et al., 1988; Yániz et al., 2010; Azwi, 2012).
The objectives of this study were to determine the efficiency of antibiotics in controlling bacterial growth on cooled and thawed ram sperm, and their potential effects on sperm viability after cooling and thawing.
Materials and methods
Sperm Processing and Evaluation
Five 3-4 years old sexually mature Corriedale rams were used as sperm donors, having the follow sperm characteristics: total sperm motility of at least 70%; and score (0-5) of sperm vigor equal or greater than 3. The rams were kept under semi-extensive conditions at the Universidade Federal de Pelotas (UFPel: 31°48’17.91″S; and 52°25’4.36″W), having access to native pasture, mineral salt and water ad libitum during the daytime and being fed concentrated supplements twice a day. At night, the rams were housed in barns covered with wood shaving bedding.
Prior to sperm collection, the preputial ostium was cleaned with sterile saline (0.9% NaCl) and paper towel. Then, a sample of the preputial content was collected with a swab. Sperm collection was performed using an artificial vagina, twice a week for six weeks. All experimental procedures used in this study were approved by the UFPel Ethics and Animal Experimentation Committee (Registration No. 4383/2010).
Sperm samples were submitted to microbiological evaluation prior to dilution with tris-egg yolk extender (Evans & Maxwell, 1987). After dilution, samples with 2 x 109 spermatozoa/mL (determined in a Neubauer chamber) were pooled, so each ram contributed equally to the final sperm concentration. Sperm samples were subsequently diluted in an extender containing: 300 mM Tris-hydroxymethyl-aminomethane; 30 mM fructose; and 94.7 mM citric acid monohydrate (Evans & Maxwell, 1987). After that, sperm samples with concentration of 4 x 108 spermatozoa/mL were split in the following treatments: Control, with no antibiotics; PES, consisting of 100,000 IU/mL penicillin and 100 µg/mL streptomycin (Milli-Q water diluted penicillin g benzathine and streptomycin sulphate, Sigma Chemical Company, St. Louis, MO, USA) (Salamon and Maxwell. 2000); GTLS, consisting of 500 µg/mL gentamicin (Gentrin, Ourofino Agronegócio, São Paulo, SP, Brazil), 100 µg/ml tylosin (Tylan 200, Elanco Animal Health, São Paulo, SP, Brazil), 300 µg/mL lincomycin and 600 µg/mL spectinomycin (LincoSpectin, Pfizer Animal Health, São Paulo, SP, Brazil) (Lorton and Sulivan, 1988); CEF, consisting of 50 µg/mL ceftiofur sodium (Excenel, Pfizer Animal Health, São Paulo, SP, Brazil) (Guilmoto and Regault, 1996) and; ENR, consisting of 1000 µg/mL enrofloxacin (Kinetomax, Bayer Animal Health, Rio de Janeiro, Brazil) (Tomczyk and Minta, 2002). All treatments were also tested at 50, 75, 125 and 150% of the recommended dose (considered as 100%). Thus, considering the control, 21 treatments were tested. Glycerol (Sigma-Aldrich, St. Louis, MO, USA) was added to the extender, at a final concentration of 5%. For each treatment, five 0.25 mL straws containing 50 x 106 spermatozoa were cooled at 5 °C for 2 h and subsequently frozen in vapor of liquid nitrogen (Salamon and Maxwell, 2000). Samples were thawed in water bath at 37o.C during 30 s.
Parameters of sperm quality were evaluated after 2 h of cooling at 5 °C and also after thawing, keeping the samples incubated in a water bath at 37 °C for 10 min. Sperm motility was determined with an optical microscope equipped with heating stage at 37 °C (Olympus BX 51, Sapporo, Japan). Sperm membrane and acrosome integrity were both determined with an epifluorescence microscope (Eclipse 80i, Nikon Instruments Inc., Melville, NY, USA). Carboxifluorescein diacetate and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) were used to evaluate membrane integrity (Harrison and Vickers, 1990), whereas propidium iodide and lectin from Arachis hypogaea conjugate (Sigma-Aldrich, St. Louis, MO, USA) were used to evaluate acrosome integrity (Kawamoto et al., 1999).
Microbiological Evaluation and Antibiogram
Microbiological evaluation was done to determine the bacteria present in samples from the preputial ostium, in the extender with no antibiotics and in fresh, cooled and thawed sperm. The samples (swabs) were streaked onto agar with 5% sheep blood and incubated at 37 °C in aerobiosis for 48 h. After incubation, the colonies were visualized and a Gram stain performed (Maza et al., 2004). The different types of colonies were isolated and incubated for 24 h in 10 mL tubes with Brain Heart Infusion (BHI) medium (Acumedia, São Paulo, SP, Brazil). Then, the culture were spread in the surface of the culture media with a Drigalski loop in the Petri plates containing the following media Brain Heart Infusion Agar (BHI, Acumedia®), Chapman (Acumedia®) and MacConkey (Acumedia®), in duplicate, respectively. The plates were incubated at 37 ºC for 24 h. Then bacteria growth (colony forming units, CFU/mL) counting was carried out. To evaluate the total bacteria numbers we consider the growth that was record in BHI, for Staphylococcithe growth in Chapman, and for Enterobacteriaceae the growth in MacConkey media. The cultures were submitted to carbohydrate battery and Gram stain, respectively (Carter, 1988).
The number of CFU/mL was quantified in sperm samples collected at the end of the cooling period and after freezing-thawing. A 100 µL aliquot of diluted sperm was streaked onto BHI agar with 5% sheep blood and incubated at 37 °C for 48 h, as described above.
An antibiogram was performed using autoclave-sterilized paper filter disks impregnated with 2µL of the tested antibiotics (CLSI, 2009). The antibiotic concentrations in the disks were: 10µg penicillin and 40µg streptomycin; 88µg gentamicin; 40µg tylosin; 100µg lincomycin and 200µg spectinomycin; 100µg ceftiofur sodium; and 200µg enrofloxacin. The disks were distributed in Mueller-Hinton agar plates (Acumedia) previously streaked with the isolated colonies. Plates were kept in an incubator at 37 °C for 24 h and the inhibition halos were measured (CLSI, 2009).
Statistical Analysis
The Shapiro-Wilk test indicated that no responses of interest followed normal distributions. Therefore, sperm motility and integrity of sperm membrane and acrosome were transformed to the arcsine scale, and the number of CFU/mL was transformed to the logarithmic scale. All parameters were compared across treatments by analysis of variance, with comparisons of means by Tukey’s test. The results referring to parameters of sperm quality were reported in their original scale for the sake of interpretation. All statistical analyses were conducted with (Statistix®, 2009).
Results
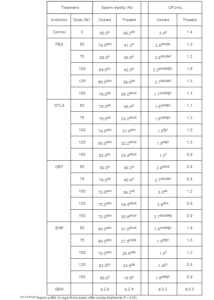
For fresh sperm, motility was 80.3 ± 1.1%, membrane integrity was 56.8 ± 6.5% and acrosome integrity was 76.6 ± 23.9%. After cooling at 5 ºC for 2 h, motility was 70.9 ± 3.0 %, membrane integrity was 69.5 ± 1.7% and acrosome integrity was 82.5 ± 2.3 %. Membrane and acrosome integrity did not differ across treatments (P > 0.05) for cooled sperm. However, even though motility of cooled sperm was generally similar among treatments, it was lower for 150% GTLS and for ENR at both 125% and 150% (P0.05), with concentrations range between 50-125%. However, at 150%, all three showed a reduced CFU/mL compared with the others concentrations and control (Table 1). No difference in CFU/mL was observed for CEF (P>0.05), among the tested concentrations.
After thawing, sperm motility was 33.4 ± 2.3%, membrane integrity was 18.2 ± 1.1% and acrosome integrity was 73.5 ± 2.3 %. Sperm motility for PES, GTLS and CEF was similar (P>0.05) to that of the control group (Table 1). However, with ENR at doses greater than 0.5µg/mL, sperm motility was reduced as compared to the control group (P<0.05). After thawing, the integrity of sperm membrane and acrosome, and the number of CFU/mL did not differ among treatments (P<0.05).
Genera such as, Staphylococcus sp., Klebsiella sp., Corynebacterium sp. and Bacillus sp. were identified in samples from fresh sperm and preputial ostium. Bacterial growth was not significant (less than 30 CFU/mL) in samples of extenders without antibiotics. In the antibiogram, Staphylococcus sp. and Klebsiella sp. presented resistance to tylosin and to the penicillin and streptomycin association.
Table 1: Motility and Colony Forming Units (CFU)/mL x 103 of Cooled and Thawed Ram Sperm with and without Inclusion of Antibiotics in the Cryopreservation Extender
*PES = 100,000 IU/ml penicillin and 100 µg/ml streptomycin; GTLS: 500 µg/ml gentamicin; 100 µg/ml tylosin; 300 µg/ml
lincomycin and 600 µg/ml spectinomycin; CEF: 50 µg/ml ceftiofur sodium; ENR: 1,000 µg/ml enrofloxacin.
Discussion
To our knowledge this is the first study that evaluated the effects of the addition of ENR and CET in extenders for ram sperm cryopreservation, along with other antibiotics, on sperm viability and bacterial counts. The microorganisms isolated in this study were usually isolated from the environment (Klebsiella sp., Corinebacterium sp., Bacillus sp. and Staphilococcus sp.), being the latter among the most common bacterial isolated from rams (Yániz et al., 2010).
The numbers of CFU/mL observed in samples from the preputial ostium and from fresh sperm were both lower than the levels recommended by the World Organization for Animal Health (OIE, 2009). However, the sperm dilution effect might play a role in the CFU/mL observed in fresh sperm, since sperm concentration is greater for rams than for bulls. Nevertheless, the hygiene conditions during sperm collection in the present study may be considered at most satisfactory, if compared with commercial SCPCs. Thus, our results suggest that ram sperm can be frozen without inclusion of antibiotics in the extender, since the freezing-thawing process by itself can reduce the bacterial population up to levels accepted by international regulatory agencies.
The antibiogram identified resistance of Staphylococcus sp. and Klebsiella sp. to the penicillin and streptomycin association and to tylosin. Despite that, tylosin contributed to inhibiting bacterial growth in cooled sperm, when associated with other antibiotics. The association of penicillin and streptomycin was effective in controlling bacterial growth in cooled sperm only at its greatest concentration. This finding reinforces the fact that there is bacterial resistance to that antibiotic association at commonly used doses. However, the post-thawing number of CFU/mL in ram sperm was similar among treatments with and without inclusion of antibiotics, regardless of the dose. The reduction in CFU/mL observed in the control treatment without antibiotics is likely to be a consequence of freezing sperm using an extender with 5% glycerol, since bacteria are usually cryopreserved with glycerol concentrations exceeding 15% (Hubálek, 2003). Reduction in the number of CFU/mL attributed to freezing was also reported with the use of 10% dimethyl sulfoxide as cryoprotectant (Kipp et al., 2004). So, it may be suggested that the reduction in CFU/mL is due to the freezing-thawing process.
The inclusion of antibiotics in extenders at the tested concentrations did not influence the integrity of either sperm membrane or acrosome, in both cooled and thawed sperm. Nevertheless, sperm motility was negatively affected with antibiotic addition. 100,000 IU/mL and 100µg/mL PES, 750µg/mL, 150µg/mL, 450µg/mL and 900µg/mL GTLS and ENR at both 1,250µg/mL and 1,500µg/mL presented sperm motility lower than the control group and also lower than that in treatments with 75,000 IU/mL and 75 µg/mL PES and 25µg/mL CEF. For frozen-thawed sperm, most treatments presented similar motility, except those where ENR was included at doses exceeding 0,500µg/mL, which reduced sperm motility. Although the present study was the first to report direct effects of ENR in the viability of cryopreserved ram sperm, other studies reported reduction in sperm motility for other species, related to impairment of spermatogenesis due to parenteral application of ENR (Aral et al., 2008; Sinha et al., 2012).
In the present study, the reduction in sperm motility may be attributed to the reduction of mitochondrial activity in spermatozoa, since quinolone antibiotics inhibit DNA gyrase (Sárközy, 2001). This particular mechanism of action allows the elimination of strains resistant to antibiotics which act on the cell wall, the cytoplasmic membrane or on protein synthesis. The development of resistance to their use was only observed under experimental conditions, after repeated passages, appearing in a slow and gradual manner (Scheer, 1987b).
Conclusions
Bacterial growth can be reduced in cooled ram sperm with inclusion of antibiotic associations of penicillin and streptomycin, and of gentamicin, tylosin, lincomycin and spectinomycin in the extender, but not with sodium ceftiofur. The inclusion of antibiotics in extenders for frozen ram sperm may not be necessary, when using healthy sperm donors under satisfactory hygiene conditions. The freezing process may keep sperm contamination within the levels recommended by regulatory health agencies. The use of enrofloxacin should be avoided due to negative effects on sperm motility.
Acknowledgements
This research was funded with a scholarship given to the first author by CAPES and with a research grant # 578597/2008-0 from MCT/CNPq/MAPA/SDA.
References
Aral, F., Karaçal, F. & Baba, F. (2008). “The Effect of Enroflocacin on Sperm Quality in Male Mice,” Research in Veterinary Science (84) 95-99.
Publisher – Google Scholar
Azawi, O. I. & Ismaeel, M. A. (2012). “Influence of Addition of Different Antibiotics in Semen Diluent on Viable Bacterial Count and Spermatozoal Viability of Awassi Ram Semen,” Veterinary World (5) 75-79.
Publisher – Google Scholar
Bielanski, A. (2007). “Disinfection Procedures for Controlling Microorganisms in the Semen and Embryos of Humans and Farm Animals,” Theriogenology (68) 1-22.
Publisher – Google Scholar
Carter, G. R. (1988). ‘Essentials of Veterinary Bacteriology and Mycology,’ 1st Ed. Ed. Roca Ltda, São Paulo, SP, Brazil.
CLSI. (2009). Clinical and Laboratory Standards Institute. ‘Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard,’ 10th Ed., Wayne, PE, USA.
de la Maza, L. M., Pezzlo, M. T., Shigei, J.T. & Peterson, E. M. (2004). ‘Color atlas of Diagnostic Microbiology,’ ASM Press, Washington D.C., USA.
Evans, G. & Maxwell, W. M. C. (1987). ‘Salamon’s Artificial Insemination of Sheep and Goats,’ Star Printery Pty Ltd, Australia.
Guilmoto, H. & Regault, M. (1996). ‘Use of Ceftiofur in Boar Semen Diluents,’ Conference Proceeding of International Pig Veterinary Society Congress 14 (1996) 138.
Harrison, R. A. P. & Vickers, S. E. (1990). “Use Fluorescent Probes to Assess Membrane Integrity in Mammalian Spermatozoa,” Journal of Reproduction and Fertility (88) 343-352.
Publisher – Google Scholar
Hubálek, Z. (2003). “Protectants Used in Cryopreservation of Microorganisms,” Cryobiology (46) 205-229.
Publisher – Google Scholar
Kawamoto, A., Ohashi, K., Kishikawa, H., Zhu, L.- Q., Azuma, C. & Murata, Y. (1999). “Two-Color Fluorescence Staining of Lectin and Anti-CD46 Antibody to Assess Acrosomal Status,” Fertility and Sterility (71) 497-501.
Publisher – Google Scholar
Kipp, F., Linnemann, E., Fisher, R. J., Sibrowski, W. & Cassens, U. (2004). “Cryopreservation Reduces the Concentration of Detectable Bacteria in Contaminated Peripheral Blood Progenitor Products,” Transfusion (44) 1098-1103.
Publisher – Google Scholar
Lorton, S. P., Sullivan, J. J., Bean, B., Kaproth, M., Kellgren, H. & Marshall, C. (1988). “A New Antibiotic Combination for Bovine Semen,” Theriogenology (29) 593-614.
Publisher – Google Scholar
Moustacas, V. S., Xavier, M. N., Carvalho Junior, C. A., Costa, E. A., Henry, M. & Santos, R. L. (2010). “Effect of Extender Supplementation with Various Antimicrobial Agents on Viability of Brucella Ovis and Actinobacillus Seminis in Cryopreserved Ovine Semen,” Theriogenology (74) 1476-1481.
Publisher – Google Scholar
OIE. (2008). World Organization for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th Ed
Publisher
Salamon, S. & Maxwell, W. M. C. (2000). “Storage of Ram Semen,” Animal Reproduction Science (62) 77-111.
Publisher – Google Scholar
Sárközy, G. (2001).” Quinolones: A Class of Antimicrobial Agents,” Veterinarni Medicina (46) 257-274.
Publisher – Google Scholar
Shin, S. J., Lein, D. H., Patten, V. H. & Ruhnke, H. L. (1988). “A New Antibiotic Combination for Frozen Bovine Semen I. Control of Mycoplasmas, Ureaplasmas, Campylobacter Fetus Ssp. Venerealis and Haemophilus Somnus in Frozen Bovine Semen,” Theriogenology (29) 577-591.
Publisher – Google Scholar
Sinha, C., Yadav, S., Yadav, B. & Singh, K. (2012). “Effects of Enrofloxacin Administration on Semen Quality of Barbari Bucks,” Journal of Advanced Veterinary Research (2) 179-183.
Publisher – Google Scholar
Thibier, M. & Guerin, B. (2000). “Hygienic Aspects of Storage and Use of Semen for Artificial Insemination,” Animal Reproduction Science (62) 233-251.
Publisher – Google Scholar
Tomczyk, G. & Minta, Z. (2002). “Elimination of Mycoplasma from the Turkey Semen,” Bulletin of the Veterinary Institute in Puławy (46) 11-15.
Publisher – Google Scholar
Yániz, J. L., Marco-Aguado, M. A., Mateos, J. A. & Santolaria, P. (2010). “Bacterial Contamination of Ram Semen, Antibiotic Sensitivities and Effects on Sperm Quality during Storage at 15°C,” Animal Reproduction Science (122) 142-149.
Publisher – Google Scholar