Discussion
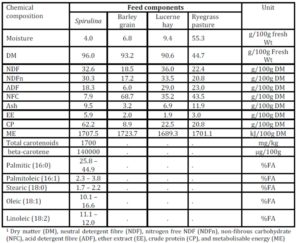
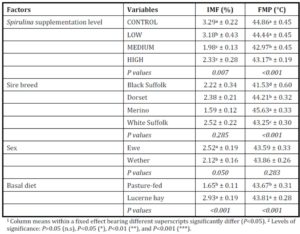
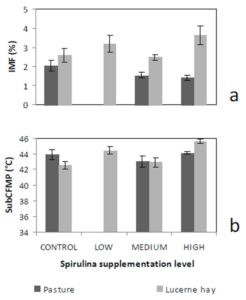
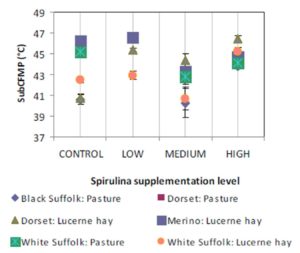
The observation of simultaneous decreases in IMF and FMP as the level of Spirulina supplementation increased from LOW to MEDIUM when compared to the unsupplemented group of lambs (CONTROL), is consistent with similar observations in another experiment where lambs were supplemented with degummed crude canola oil (Flakemore et al. 2014a, 2014b).The observed decrease in FMP suggests a possible increase in unsaturated fatty acids (UFA) and a decrease in saturated fatty acids (SFA) and softer fat probably due to Spirulina’s naturally high beta-carotene content (Table 1; Holman & Malau-Aduli, 2013). Retinoic acid, a metabolite of beta-carotene, plays a role in ruminant stearoyl Co-A desaturase enzyme activity and expression that drives SFA desaturation in the rumen (Siebert et al., 2000). These changes in SFA/UFA differ from supplementation trials involving Spirulina fed to rabbits (Peiretti & Meineri, 2011). However, rabbits are pseudo-ruminants, unlike lambs, where dietary UFA in Spirulina is relatively unprotected from biohydrogenation by rumen microflora.
Dietary intake has a marked effect on ruminant IMF. During periods of low dietary intake, there is a marked reduction in IMF compared to periods of high dietary intake (Geesink & Zerby, 2010, Hopkins et al., 2007, Roberts et al., 2007). This study’s findings, however, diverge from this preconception. This may have arisen from our experimental design in which Lucerne hay fed lambs were confined to stalls. This confinement would have restricted energy expenditure to well below that of equivalent pasture run lambs. We suggest this difference in energy utilisation would be reflected in our IMF findings.
High FMP is associated with elevated SFA and lowered UFA concentrations. Dietary factors influence ruminant adipose deposits in terms of fatty acid composition (Webb & O’Neill, 2008), although to a lesser extent than in monogastrics (Bas & Morand-Fehr, 2000). Dietary fatty acids influence adipose fatty acid composition and this influence is limited by rumen biohydrogenation of UFA (Bas & Morand-Fehr, 2000). However, linolenic acid, a fatty acid associated with pastures, is relatively resistant to this biohydrogenation compared to other fatty acids (Bas & Morand-Fehr, 2000). Furthermore, dietary linolenic acid has been reported to increase ruminant UFA levels, such as eicosapentaenoic acid, docosahexaenoic acid and docosapentaenoic acid (Raes et al., 2004). Subsequently, lambs fed pasture basal diets are expected to have greater UFA and lower FMP compared to those fed Lucerne hay. Aurousseau et al. (2007) observed similar results with lambs finished on both pasture and concentrates where UFA was lower in pasture-fed lambs than in their stall fed counterparts on concentrates.
IMF is influenced by nutrition, particularly dietary lipid content. This premise would explain the observed differences between MEDIUM and HIGH Spirulina supplementation levels with Lucerne hay basal diet. However, our findings with ryegrass basal diets are not easily explainable because of the tendency of dietary lipid to be partitioned more towards subcutaneous fat deposition than IMF (Kempster, 1981). Holman et al. (2012) reports of an increase in lamb body condition score with increased Spirulina supplementation level in these same lambs. However, body condition score is a subjective assessment of the degree of subcutaneous fat deposition. Hence, it is possible that the dietary lipid sourced from Spirulina was partitioned primarily to the subcutaneous fat depot. Therefore, Spirulina supplementation with ryegrass basal diet resulted in leaner lamb meat compared to CONTROL lambs.
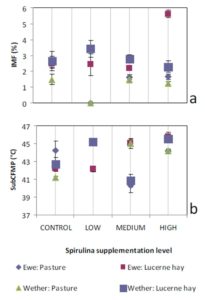
Ruminant sire breed effects on IMF (McPhee et al., 2008, Pethick et al., 2010) and FMP (Aurousseau et al., 2007, Costa et al., 2009, Malau-Aduli et al., 2000) have been widely reviewed previously. The influences of sex on IMF have also been reported by Dervishi et al., (2012), McPhee et al., (2008) and Pethick et al., (2010). Our findings align with these observations.
Acknowledgements
The Australian Wool Education Trust (AWET) and The University of Tasmania (UTAS) funded this research with grants and postgraduate top-up scholarships for which the authors are grateful to both AWET and UTAS. The contributions of Chris Gunn, Barrie Wells, Florence Malecot, Loudella Deladerriere, John Otto and Will Bignell during the sheep breeding, feeding trials and laboratory analysis are highly appreciated.
Conclusion
Lambs grazed on a basal diet of ryegrass had significantly lower IMF and FMP compared to their counterparts fed Lucerne hay. As the level of Spirulina supplementation of lambs increased from LOW to MEDIUM, there were simultaneous decreases in IMF and FMP in comparison with unsupplemented lambs. Ewe lambs had significantly higher IMF than wether lambs, but sex differences in FMP were negligible.
References
1. AOCS – American Oil Chemists’ Society (2009). Slip melting point ISO Standard. AOCS Official Method Cc 3b-92.
2. Aurousseau, B., Bauchart, D., Galot, A.L., Prache, S., Micol, D. & Priolo, A., (2007). Indoor fattening of lambs raised on pasture: 2. Influence of stall finishing duration on triglyceride and phospholipid fatty acids in the longissimus thoracis muscle. Meat Science, 76: 417-427.
Publisher – Google Scholar
3. Bas, P. & Morand-Fehr, P., (2000). Effect of nutritional factors on fatty acid composition of lamb fat deposits.Livestock Production Science, 64: 61-79.
Publisher – Google Scholar
4. Costa, R.G., Malveira Batista, A.S., de Azevedo, P.S., Ramos do Egypto Queiroga, R.d.C., Madruga, M.S. & de Araujo Filho, J.T., (2009). Lipid profile of lamb meat from different genotypes submitted to diets with different energy levels. Brazilian Journal of Animal Science, 38: 532-538.
5. Dervishi, E., Joy, M., Alvarez-Rodriguez, J., Serrano, M. & Calvo, J.H., (2012). The forage type (grazing versus hay pasture) fed to ewes and the lamb sex affect fatty acid profile and lipogenic gene expression in the longissimus muscle of suckling lambs. Journal of Animal Science, 90: 54-66.
Publisher – Google Scholar
6. Flakemore, A.R., McEvoy, P.D., Balogun, R.O., Malau-Aduli, B.S., Nichols, P.D. & Malau-Aduli, A.E.O., (2014a).Degummed crude canola oil supplementation affects fat depot melting points in purebred and first-cross Merino sheep. Animal and Veterinary Sciences, 2: 75-80.
Publisher – Google Scholar
7. Flakemore, A.R., Balogun, R.O., McEvoy, P.D., Malau-Aduli, B.S., Nichols, P.D. & Malau-Aduli, A.E.O., (2014a). Genetic variation in intramuscular fat of prime lambs supplemented with varying concentrations of degummed crude canola oil. International Journal of Nutrition and Food Sciences, Vol. 3, Issue 3, (In Press).
Google Scholar
8. Folch, J., Lees, M. & Sloane-Stanley, G.H., (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226: 497-509.
Google Scholar
9. Holman, B.W.B., Kashani, A. & Malau-Aduli, A.E.O., (2012). Growth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospira platensis) supplementation. American Journal of Experimental Agriculture, 2: 160-173.
Publisher – Google Scholar
10. Holman, B.W.B. & Malau-Aduli, A.E.O., (2013). Spirulina as a livestock supplement and animal feed. Journal of Animal Physiology and Animal Nutrition, 97: 615-623.
Publisher – Google Scholar
11. Hopkins, D.L., Stanley, D.F., Toohey, E.S., Gardner, G.E., Pethick, D.W. & van de Ven, R., (2007). Sire and growth path effects on sheep meat production 2. Meat and eating quality. Australian Journal of Experimental Agriculture, 47: 1219-1228.
12. Kempster, A.J., (1981). Fat partition and distribution in the carcasses of cattle, sheep and pigs: A review. Meat Science, 5: 83-98.
Publisher – Google Scholar
13. Malau-Aduli, A.E.O., Edriss, M.A., Siebert, B.D., Bottema, C.D.K. & Pitchford, W.S., (2000). Breed differences and genetic parameters for melting point, marbling score and fatty acid composition of lot-fed cattle. Journal of Animal Physiology and Animal Nutrition, 83: 95-105.
Publisher – Google Scholar
14. McPhee, M.J., Hopkins, D.L. & Pethick, D.W., (2008). Intramuscular fat levels in sheep muscle during growth. Australian Journal of Experimental Agriculture, 48: 904-909.
Publisher – Google Scholar
15. Peiretti, P.G. & Meineri, G., (2011). Effects of diets with increasing levels of Spirulina platensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livestock Science, 140: 218-224.
Publisher – Google Scholar
16. Raes, K., De Smet, S. & Demeyer, D., (2004). Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: a review. Animal Feed Science and Technology, 113: 199-221.
Publisher – Google Scholar
17. Roberts, A.J., Paisley, S.I., Geary, T.W., Grings, E.E., Waterman, R.C. & MacNeil, M.D., (2007). Effects of restricted feeding of beef heifers during the postweaning period on growth, efficiency, and ultrasound carcass characteristics.Journal of Animal Science, 85: 2740-2745.
Publisher – Google Scholar
18. SAS, (2009). Statistical Analysis System. SAS Institute, Cary, North Carolina, USA, version 9.2.
19. Siebert, B.D., Pitchford, W.S., Kuchel, H., Kruk, Z.A. & Bottema, C.D.K., (2000). The Effect of β-carotene on desaturation of ruminant fat. Asian-Australasian Journal of Animal Science, 13: 185-188.
20. Webb, E.C. & O’Neill, H.A., (2008). The animal fat paradox and meat quality. Meat Science, 80: 28-36.
Publisher – Google Scholar
21. Woods, V.B. & Fearon, A.M., (2009). Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livestock Science, 126: 1-20.
Publisher – Google Scholar