Antibiotic sensitivity tests were performed using a standard disk diffusion method and the results interpreted as per NCCLS guidelines (Quinn et al., 1994). The disks used were: ampicillin, cephalothin, chloramphenicol, clindamycin, erythromycin, gentamicin, neomycin, penicillin, tetracycline, trimethoprim-sulfa, and vancomycin.
Results
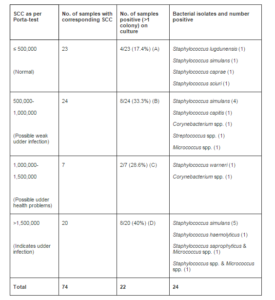
Of a total of 74 udder half samples subjected to Porta-test, 23 were negative for high SCC, and 4/23 (17.4%) were culture-positive (Table 1). Of the 51 porta-test- positive samples, consisting of 3 categories, 35.2% were positive in culture. The differences in culture positivity in the 4 different categories of SCC, however were not statistically significant (p=0.41).
The most common bacterial species among the 24 isolates recovered was Staphylococcus simulans (10/24) (42%). Other isolates identified to species level were: S. capitis (1), S. caprae (1), S. haemolyticus (1), S. lugdunensis (1), S. saprophyticus (1), S. sciuri (1), and S. warneri (1).
Of 24 bacterial isolates tested against 12 antimicrobial drugs, only 2 isolates showed resistance. These were: one S. sciuri isolate resistant to penicillin and erythromycin, and one isolate of S. capitis resistant to tetracycline.
Discussion
There is a place in caprine herd health management for goat side tests, as an increase in SCC is an indication of inflammation. If this is detected early and the factors contributing to the elevated SCC are addressed, it would be reasonable to expect that production would be increased. In comparison to the California Mastitis Test, the Porta-test is much more user friendly test that does not require as much training to perform, and therefore there is less subjectivity in the interpretation of the results. Also, antibiotics do not interfere with the test. This would be a more appropriate test than the conventional California Mastitis Test for “on farm” use. A study by Kretschmer et al. (2008) showed that Porta-test can be used as an on-farm tool for determining udder health status in sheep as either “healthy” or within the “chronic/clinical mastitis” range. Although it is a convenient and novel tool, Porta-test was not able to differentiate between udder sides between “healthy” and “infection/subclinical mastitis” ranges in sheep. Our results show that it cannot be relied upon as the sole test of caprine udder health in the context of subclinical bacterial mastitis. According to Smith and Sherman (2009), tests other than culture, including SCC determination are unsatisfactory in goats. In this regard, it may be noted that in a study using repeated testing of sheep milk samples during a 10 week period, Hariharan et al., (2004) found no association between SCC and culture positivity. Studies on dairy goats in Switzerland (Schaeren and Maurer, 2006), and the United States (Min et al., 2007) also showed that tests for SCC are of limited value for diagnosis of intramammary infection in goats. These observations, however, contradict the findings of a recent study, which concluded that SCC measurements can be used for the prediction of udder infection in dairy goats (Persson and Olofsson, 2011). Of note, factors other than bacterial infection, such as caprine arthritis and myeloencephlitis virus infection and consumption of avocado (Persea Americana) leaves can cause inflammation of the caprine mammary gland, and increase in SCC (Lerondelle et al., 1992, Smith and Sherman, 2009). There are several non-infectious variation factors of SCC, and these include dietary and medical stresses, and physiological factors such as the stage of lactation, and estrus in goats (Bergonier et al., 2003, Luengoet al., 2004). The inflammatory and immune processes of the caprine udder are not fully understood, and need further research.
The most frequently isolated bacterial species in this study was Staphylococcus simulans, which is one of the coagulase-negative staphylococci that can be involved in subclinical mastitis in goats (Bergonier et al., 2003, Berry, 2006). Coagulase-negative staphylococci have been found to be the most common organisms isolated from subclinical caprine mastitis in different parts of the world (Contreras et al., 1995, Moroni et al., 2005, Hall and Rycroft, 2007)
Antimicrobial drug resistance among the isolates in this study was minimal or negligible. In contrast, a study of isolates from goats with subclinical mastitis in Italy (Virdis et al., 2010) showed presence of multi-drug resistantStaphylococcus aureus as well as coagulase negative staphylococci. A study in Switzerland showed that in general, compared to S. aureus, coagulase-negative staphylococci from goats and sheep have less resistance to penicillin and ampicillin (Kunz et al., 2011).
In summary, the results of Port-test and culture show that the diagnosis of sub-clinical bacterial mastitis in the caprine cannot be extrapolated with the sole use of Porta- test. However, a high SCC along with culture positivity may indicate intramammary infection. The bacterial isolates and their respective sensitivities in this study were consistent with the findings in other regions of the world.
References
Bergonier, D., de Crémaux, R., Rupp, R., Lagriffoul, G. & Berthelot, X. (2003). “Mastitis in Small Dairy Ruminants,”Veterinary Research 34: 689-716.
Publisher – Google Scholar
Berry, E. (2006). “Update on Caprine Mastitis,” Goat Veterinary Society Journal. 22: 40-42.
Publisher
Contreras, A., Corrales, J. C., Sierra, D. & Marco J. (1995). “Prevalence and Aetiology of Non-Clinical Intramammary Infection in Murciano-Granadina Goats,” Small Ruminant Research 17: 71-78.
Publisher – Google Scholar
Hall, S. M. & Rycroft, A. N. (2007). “Causative Organisms and Somatic Cell Counts in Subclinical Intramammary Infections in Milking Goats in the UK,” Veterinary Record 160: 19-22.
Publisher – Google Scholar
Hariharan, H., Donachie, W., Macaldowie, C. & Keefe, G. (2004). “Bacteriology and Somatic Cell Counts in Milk Samples from Ewes on a Scottish Farm,” Canadian Journal of Veterinary Research 68:188-192.
Publisher – Google Scholar
Kretschmer, E. R., Holcombe, D. W., Fernandez, G. C. J. & Redelman, D. (2008). ‘Efficacy of the PoraSCC® Milk Test to Estimate Somatic Cell Count (SCC) and Effect of Sampling Day on Constituents in Sheep Milk,’ Proceedings, Western Section, American Society of Animal Science 59: 281-284.
Google Scholar
Kunz, F., Corti, S., Giezendanner, N., Stephan, R., Wittenbrink, M. & Zweifel, C. (2011). “Antimicrobial Resistance of Staphylococcus Aureus and Coagulase Negative Staphylococci Isolated from Mastitis Milk Samples from Sheep and Goats,” Schweizer Archiv Fur Tierheilkunde 153: 63-69.
Publisher – Google Scholar
Lerondelle, C., Richard, Y., & Issartial, J. (1992). “Factors Affecting Somatic Cell Counts in Goat Milk,” Small Ruminant Research 8:129-139.
Publisher – Google Scholar
Leslie, K., Baratt, K., Petersson, C. & Bashiri, A. (2006). “An Evaluation of the PortaSCC® Test as a Measure of Udder Health Status of Dairy Cows (An Excerpt from a Technical Report),” http://www.portacheck.com/guelph.php (Accessed 10/15/2013).
Publisher
Luengo, C., Sánchez, A., Corrales, J. C., Fernández, C. & Contreras, A. (2004). “Influence of Intramammary Infection and Non-Infection Factors on Somatic Cell Counts in Dairy Goats,” Journal of Dairy Research 71: 169-174.
Publisher – Google Scholar
Min, B. R., Tomita, G. & Hart, S. P. (2007). “Effect of Subclinical Intramammary Infection on Somatic Cell Counts and Chemical Composition of Goat’s Milk,” Journal of Dairy Research 74:204-210.
Publisher – Google Scholar
Moroni, P., Pisoni, G., Antonini, M., Ruffo, G., Carli, S., Varisco, G. & Boettcher, P. (2005). “Subclinical Mastitis and Antimicrobial Susceptibility of Staphylococcus Caprae and Staphylococcus Epidermidis Isolated from Two Italian Goat Herds,” Journal of Dairy Science 88: 1694-1704.
Publisher – Google Scholar
Oliszewski, R., Nunez de Kairuz, M., Gonzalez, S. & Oliver, G. (2004). “Beta-glucuronidase method to Determine Mastitis Levels in Goat Milk,” In: Methods in Molecular Biology, vol. 268: Public Health Microbiology: Methods and Protocols. Spencer, J. F. T. & Ragout de Spencer, A. L. (Eds.) Humana Press Inc., Totowa, N.J., USA. 475-479.
Publisher
Persson, Y. & Olofsson, I. (2011). “Direct and Indirect Measurement of Somatic Cell Count as Indicator of Intramammary Infection in Dairy Goats,” Acta Veterinaria Scandinavica 53:15.
Publisher – Google Scholar
Petersson, K. H., Connor, L. A., Petersson-Wolfe, C. S. & Rego, K. A. (2011). “Short Communication: Evaluation of Confirmatory Stains Used for Direct Microscopic Somatic Cell Counting of Sheep Milk,” Journal of Dairy Science 94: 1908-1912.
Publisher – Google Scholar
Philpot, W. N. (1984). Mastitis Management, 2nd edition. Babson Bros. Co., Oak Brook, Illinois, USA. 5-6.
Publisher
Quinn, P. J., Carter, M. E., Markey, B. K. & Carter, G. R. (1994). ‘Clinical Veterinary Microbiology,’ Wolfe/Mosby Publishing, London, UK. 49-335.
Google Scholar
Ruegg, P. L. & Rienemann, D. J. (2002). “Milk Quality and Mastitis Tests,” University of Wisconson, Madison. http://milkquality.wisc.edu/wp-content/uploads/2011/…
Publisher – Google Scholar
Schaeren, W. & Maurer, J. (2006). “Prevalence of Subclinical Udder Infection and Individual Somatic Cell Counts in Three Dairy Goat Herds during a Full Lactation,” Schweizer Archiv Fur Tierheilkunde. 148:641-648.
Publisher – Google Scholar
Smith, M. C. & Sherman, D. M. (2009). Goat Medicine, 2nd edition, Wiley-Blackwell, Ames, Iowa. 647-689.
Publisher – Google Scholar
Virdis, S., Scarano, C., Cossu, F., Spanu, V., Spanu, C. & De Santis, E. P. L. (2010). “Antibiotic Resistance in Staphylococcus Aureus and Coagulase Negative Staphylococci Isolated from Goats with Subclinical Mastitis,”Veterinary Medicine International , 6 pages.
Publisher – Google Scholar