Acknowledgements
The authors would like to thank V. Matthew-Belmar, E. Brathwaite, J. Coughlin, Z. Pearl, B. Read, and G. Roy for technical assistance.
References
1. Annual Report. (2009). Ministry of Agriculture, Forestry and Fisheries, Grenada – Annual Report, 2009.
2. Arsenault, J., Girard, C., Dubreuil, P., Daignault, D., Galarneau, J.R., Boisclair, J., Simard, C. & Belanger, D. (2003). “Prevalence of and carcass condemnation from maedi-visna, paratuberculosis and caseous lymphadenitis in culled sheep from Quebec, Canada,” Preventive Veterinary Medicine, 59:67-81.
3. Debien, E., Helie, P., Buczinski, S., Leboeuf, A., Belanger, D. & Drolet, R. (2013). “Proportional mortality: A study of 152 goats submitted for necropsy from 13 goat herds in Quebec, with a special focus on caseous lymphadenitis,” Canadian Veterinary Journal, 54:581-587.
4. Dercksen, D.P., Brinkhof, J.M., Dekker-Nooren, T., Maanen, K., Bode, C.F., Baird, G. & Kamp, E.M. (2000). “A comparison of four serological tests for the diagnosis of caseous lymphadenitis in sheep and goats,” Veterinary Microbiology, 31: 167-175.
5. Estevao Belchior, S., Gallardo, A., Abalos, A., Diaz, Y., Alvarez, L., Callejo, R., Prieto, M., Jodor, N. & Jensen, O. (2007). Diagnosis of caseous lymphadenitis in sheep from Patagonia. Revista Argentina de Microbiologia 39: 44-46.
6. Funke, G. & Bernard, K. A. (2011). “Coryneform Gram-Positive Rods” In: Manual of Clinical Microbiology, 10th edition. Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L. & Warnock, D.W (Editors). pp. 413-442. ASM Press, Washington, DC.
7. Guimaraes, A.S., Carmo, F.B., Heinemann, M.B., Portela, R.W., Meyer, R., Lage, A.P., Sdyffert, N., Miyoshi, A., Azevedo, V. & Gouveia, A.M. (2011). “High sero-prevalence of caseous lymphadenitis identified in slaughter samples as a consequence of deficiencies in sheep farm management in the state of Minas Gerais, Brazil,” BMC Veterinary Research 2011. 7:68.
8. Harkiss, G. (2014). www.mvdiagnostics.co.uk. Accessed 14 May, 2014.
9. Hyphen Biomed. (2014). www.hyphen-biomed.com. Accessed 14 May, 2014.
10. Komala, T.S., Ramlan, M., Yeoh, N.N., Surayani, A.R. & Sharifah Hamidah, S.M. (2008). “A survey of caseous lymphadenitis in small ruminant farms from two districts in Perak, Malaysia — Kinta and Hilir Perak,” Tropical Biomedicine, 25:196-201.
11. Kumar, J., Singh, F., Tripathi, B.N., Kumar, R. & Dixit, S.K. (2012). “Epidemiological, bacteriological and molecular studies on caseous lymphadenitis in Sirohi goats of Rajasthan, India,” Tropical Animal Health and Production, 44:1319-1322.
12. Kumar, J., Tripathi, B.N., Sonawane, G.G. & Dixit, S.K. (2013). “Rapid detection of Corynebacterium pseudotuberculosis in clinical samples from sheep,” Tropical Animal Health and Production, 45:1429-1435.
13. Nogradi, N., Spier, S.J., Toth, B. & Vaughan, B. (2012). “Musculoskeletal Corynebacterium pseudotuberculosis infection in horses: 35 cases (1999-2009),” Journal of American Veterinary Medical Association, 241:771-777.
14. Peel, M.M., Palmer, G.G., Stacpoole, A.M. & Kerr, T.G. (1997). “Human lymphadenitis due to Corynebacterium pseudotuberculosis: Report of ten cases from Australia and Review,” Clinical Infectious Diseases, 24:185-191.
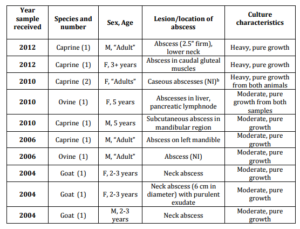
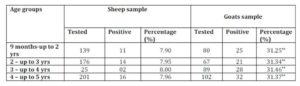
15. Personal communication. (2014). Veterinary Diagnostic Laboratory, St. George’s University, Grenada, West Indies.
16. Powell, J. (2014). “Caseous Lymphadenitis in Small Ruminants,” University of Arkansas Publication FSA3095. http://www.uaex.edu/publications/pdf/FSA-3095.pdf
17. Quinn, P.J., Carter, M.E., Markey, B. & Carter, G.R. (1994). Clinical Veterinary Microbiology, Wolfe/Mosby Publishing, London, England. pp. 137-143.
18. Reboucas, M.F., Portela, R.W., Lima, D.D., Loueiro, D., Bastos, B.L., Moura-Costa, L.F., Vale, V.L., Miyoshi, A., Azevedo, V. & Meyer, R. (2011). “Corynebacterium pseudotuberculosis secreted antigen-induced specific gamma-interferon production by peripheral blood leukocytes: Potential diagnostic marker for caseous lymphadenitis in sheep and goats,” Journal of Veterinary Diagnostic Investigation, 23:213-220.
19. Schreuder, B.E., Ter, L.E.A. & Dercksen, D.P. (1994). “Eradication of caseous lymphadenitis in sheep with the help of a newly developed ELISA technique,” Veterinary Record, 135:174-176.
20. Shpigel, N.Y., Elad, D., Yeruham, I., Winkler, M. & Saran, A. (1993). “An outbreak of Corynebacterium pseudotuberculosis infection in an Israeli dairy herd,” Veterinary Record, 133:89-94.
21. Sutherland, S.S., Ellis, T.M., Mercy, A.R., Paton, M.W. & Middleton, H. (1987). “Evaluation of an enzyme-linked immunosorbent assay for the detection of Corynebacterium pseudotuberculosis infection in sheep,” Australian Veterinary Journal, 64:263-266.
22. Washburn, K.E., Bissett, W.T., Fajt, V.R., Libal, M.C., Fosgate, G.T., Miga, J.A & Rockey, K.M. (2009). “Comparison of three treatment regimens for sheep and goats with caseous lymphadenitis,” Journal of American Veterinary Medical Association, 234:1162-1166.
23. Williamson, L.H. (2001). “Caseous lymphadenitis in small ruminants,” Veterinary Clinics of North America — Food Animal Practice, 17:359-371.
24. Windsor, P.A. (2014). “Managing control programs for ovine caseous lymphadenitis and paratuberculosis in Australia, and the need for persistent vaccination,” Veterinary Medicine: Research and Reports, 2014, 5:11-22.