Introduction
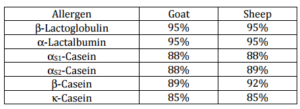
Allergy to cow’s milk (CM) protein is a major cause of food allergy in infants (Dubuisson et al., 2002). The large majority of patients with CM allergy (CMA) also react against goat and sheep’s milks (G/SM) (Restani et al., 1999). This strong cross-reactivity can be explained by the high degree of sequence homology between the main G/SM and CM allergens: β-lactoglobulin (Bos d5);α-lactalbumin (Bos d4) and caseins (αS1, αS2, β and  ) (Bos d8) (Table 1). Nevertheless, in Europe, a new population of patients has emerged since the beginning of the 1990s. These patients show, most of the time, severe allergy reactions to G/SM associated with tolerance to CM (Wüthrich et al., 1995; Umpiérrez et al., 1999; Lamblin et al., 2001; Attou et al., 2005; Martins et al., 2005; Ah-Leung et al., 2006; Viñas et al., 2012). Whereas CMA appears in the first months of life and in most of the cases disappears by the age of three, G/SM allergy appears later and is persistent (Ah-Leung et al., 2006). The role of caseins in this reactivity seems to be predominant as compared to that of β-lactoglobulin or α-lactalbumin (Umpiérrez et al., 1999; Ah-Leung et al., 2006; Muñoz Martín et al., 2004; Viñas et al., 2012). This observation is not surprising because sequence homology between bovine β-lactoglobulin and α-lactalbumin (Table 1) with goat/sheep’s corresponding proteins is very high (95%). Caseins sequences from cows and goats/sheep are more distant (85-92%) and it is therefore likely that specific epitopes in goat/sheep’s caseins are recognized by the patients with G/SM allergy (G/SMA) and tolerant to CM, however these epitopes are still unknown. Since identity between goat and sheep caseins reach 95-99%, caseins epitopes between goats and sheep are likely common between the two species. We report that the molecular basis of the reactivity to CM cheese of patients with severe G/SMA but tolerating CM is an epitope included in 1-105 peptide of bovine -casein but absent in the full-length protein.
) (Bos d8) (Table 1). Nevertheless, in Europe, a new population of patients has emerged since the beginning of the 1990s. These patients show, most of the time, severe allergy reactions to G/SM associated with tolerance to CM (Wüthrich et al., 1995; Umpiérrez et al., 1999; Lamblin et al., 2001; Attou et al., 2005; Martins et al., 2005; Ah-Leung et al., 2006; Viñas et al., 2012). Whereas CMA appears in the first months of life and in most of the cases disappears by the age of three, G/SM allergy appears later and is persistent (Ah-Leung et al., 2006). The role of caseins in this reactivity seems to be predominant as compared to that of β-lactoglobulin or α-lactalbumin (Umpiérrez et al., 1999; Ah-Leung et al., 2006; Muñoz Martín et al., 2004; Viñas et al., 2012). This observation is not surprising because sequence homology between bovine β-lactoglobulin and α-lactalbumin (Table 1) with goat/sheep’s corresponding proteins is very high (95%). Caseins sequences from cows and goats/sheep are more distant (85-92%) and it is therefore likely that specific epitopes in goat/sheep’s caseins are recognized by the patients with G/SM allergy (G/SMA) and tolerant to CM, however these epitopes are still unknown. Since identity between goat and sheep caseins reach 95-99%, caseins epitopes between goats and sheep are likely common between the two species. We report that the molecular basis of the reactivity to CM cheese of patients with severe G/SMA but tolerating CM is an epitope included in 1-105 peptide of bovine -casein but absent in the full-length protein.
Table 1: Identity of G/SM Major Allergens with their Bovine Corresponding Proteins
(alignment given identity % was done with ClustalW2 at EMBL-EBI, Cambridge, UK)
Material and Methods
Patients
Patient #816, born in 1991, presented several anaphylactic reactions after ingesting diverse cheeses. The most severe shock arose with the goat cheeses and the last one, occurring in 2008, required 4 injections of adrenalin. CM cheeses also caused symptoms but less severe, ranging from oral syndrome to asthmatic dyspnoea. The most severe reaction (asthma) with CM cheeses arose after the ingestion of Raclette (melted cow’s cheese of Swiss origin). As a child, he was never diagnosed allergic to CM. The tests are positive for GM (SPT: weal of 6 mm; IgE > 100 kUA.L-1 in 2008) and for CM (SPT: weal of 3 mm, IgE 3.24 kUA.L-1 in 2008) (Table 2). Cutaneous tests for cheeses are strongly positive for Roquefort (sheep’s milk cheese) with a weal of 15 mm but also for CM cheeses such as Camembert (weal of 6 mm) and Raclette (weal of 10 mm).
Table 2: SPT and Specific IgE
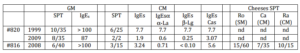
SPT: skin prick test; specific-IgE (IgEs) in kUA.L-1; cow’s milk (CM); goat’s milk (GM); sheep’s milk (SM); α-lactalbumin (α-La); β-lactoglobulin ( β-Lg); total caseins (Cas); Roquefort (made with sheep’s milk) (Ro), camembert (made with cow’s milk) (Ca) and raclette (made with cow’s milk) (Ra) cheeses.
Patient #820, born in 1993, presented two anaphylactic reactions at the age of 4 and 7, a few minutes after ingesting goat cheese. The two shocks were severe (urticaria, oedema of the face and acute asthma). As a child, he was never diagnosed allergic to CM although having presented an atopic dermatitis during his childhood (no testing was done). Nevertheless, since then, patient #820 reports allergic symptoms with CM cheeses, particularly with cheese spreads. The presented symptoms ranged from oral syndrome to asthmatic dyspnoea. The tests are positive for the GM (SPT: weal of 10 mm, IgE 87 kUA.L-1 in 2008) and for CM (PT: weal of 6 mm in 1999, negative in 2010, IgE 7.7 kUA.L-1 in 1999, 1.9 kUA.L-1 in 2009) (Table 2). We did not do any SPT for suspected cheeses.
In these two cases, G/SMA is confirmed. For the reactions imputed to CM cheeses, the interpretation was more delicate and two hypotheses were likely and perhaps not isolated from each other: 1) contamination of CM by GM; or 2) allergy to CM revealed only in its presentation under different forms of cheeses.
Serum was collected in the Laboratory of Immunology and Allergy of the Angers University Hospital (CHU) (France). Its was used under the signed consent of the patient or parents and after approval by the internal Ethical Committee of the hospital. Total IgE and specific IgE assays were done with the ImmunoCap® system (Phadia AB, Uppalla, Sweden).
Skin Prick Test
Skin Prick Test (SPT) were realized with natural cow’s milk, commercial extract of Goat milk (ALK, Hørsholm, Denmark) and natural cheeses. Prick tests with allergens are considered positive when the wheal is either equal or superior to 3 mm or equivalent to half of the wheal with histamine (positive control).
Preparation of Milk and Cheese Extracts
Goat caseins (GCN) and goat whey proteins (GW) were separated from goat’s raw milk. Milk fat was removed after centrifugation at 5000 g for 30 min at 30°C. Milk was diluted 1:3 in water and pH was adjusted at 4.6 with 1N HCl. After 1 h at room temperature with agitation the solution was centrifuged for 10min 8000 g. Caseins contained in the pellet were resuspended in water at pH 7 at room temperature. Both whey proteins contained in the supernatant and solubilized caseins were dialyzed against milli-Q water for 48 h at 4°C and lyophilized. They were dissolved in Milli-Q water just prior use at the appropriate concentration.
Cheeses (1 g) were grated and suspended in 5 mL of 0.4 M trisodium citrate buffer pH 8.3. The suspensions were homogenized for 1 min in an ice bath using an Ultra-Turrax T25 (IKA-Labortechnik, Staufen, Germany). Cheese were goat’s cheese (GC) and cow’s cheese (CC).
Western Blot
In a first step, milk and cheese proteins were separated according to their relative mass (Mr) by 12% SDS-PAGE after reduction. Milk and homogenized cheese suspension (200 mg/mL) were diluted 1:10 in SDS-PAGE loading buffer (60 mM Tris-HCl pH 6.8, 2% (w/v) SDS, 10% (v/v) glycérol, 0.025% (w/v) bromophenol blue and 5% (v/v)β -mercaptoethanol and approximately 7.5 µg of proteins were loaded on the gel. Molecular weight makers were LMW marker (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and Precision Plus Protein Standards (Biorad, Hercules, CA, USA).
After separation proteins were electro-transferred on nitrocellulose membrane (Biorad) for 1 h at 250 mA in a buffer containing 25mM Tris, 192 mM glycine, 20% (v/v) ethanol. Membrane were incubated with either monoclonal antibodies specific of each casein or with the serum of the patient. Anti-bovine casein monoclonal antibodies mAb567 anti-S1-casein (zone 149-166), mAb521 anti-S2-casein (zone 96-114), mAb657 anti- -casein (zone 133-150) and mAb257 anti-β-casein (zone 98-115) were a kind gift of Dr D. Dupont (INRA and Agrocampus, Rennes, France) (Johansson et al., 2009).
For staining with monoclonal antibodies, membranes were incubated for 1 h with a blocking solution of PBS (0.136 M NaCl, 2.68 mM KCl, 1.46 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4) containing 0.05% (v/v) Tween-20 (PBS/T) and 4% polyvinylpyrolidone (PVP) (w/v). After 2 washings for 10 min with PBS/T, membranes were incubated for 1 h with hybridoma supernatant containing the anti-casein monoclonal antibody. Membrane was washed as previously and incubated for 1 h at room temperature with an alkaline phosphatase-conjugated anti-mouse IgG (Sigma, St Quentin-Fallavier, France) diluted 1:3000 in PBS/T. The secondary antibody binding was revealed with the Immuno-Star AP kit (Biorad). Pictures were captured with a LAS-3000 imager (Fugifilm, Tokyo, Japan).
For patient’s IgE detection, membranes were treated as describe above except that monoclonal antibodies were replaced by patient’s serum diluted 1:20 in PBS/T containing 2% (w/v) PVP. Bound IgE was revealed with an horse-radish peroxidase-conjugated anti-human IgE (DAKO, Trappes, France) diluted 1:100,000 in PBS/T containing 2% (w/v) PVP by chemo-luminescence (HRP Color Development kit, Biorad). Pictures were captured every 30 sec with a LAS-3000 imager (Fugifilm, Tokyo, Japan) equipped with the appropriate filters.
Results and Discussion
CM, GM, CM cheese (line CC), GM cheese (line GC) as well as whole goat caseins (line GCN) and goat whey proteins (line GW) extracted from GM were submitted to SDS-PAGE. The gels were probed with antibodies specific for each bovine casein. The results show that all four antibodies are able to recognize goat caseins (αS1, αS2, β and  ) with good specificity (Fig. 1, see line GCN). In case of αS1- and β-caseins, many other bands are revealed. They correspond to degraded (hydrolyzed) forms of the proteins. In case of αS2- and
) with good specificity (Fig. 1, see line GCN). In case of αS1- and β-caseins, many other bands are revealed. They correspond to degraded (hydrolyzed) forms of the proteins. In case of αS2- and  -caseins only one band with lower molecular weight appears only in lines corresponding to cheeses. For -casein it can be attributed to peptide 1-105 obtained by cleavage of
-caseins only one band with lower molecular weight appears only in lines corresponding to cheeses. For -casein it can be attributed to peptide 1-105 obtained by cleavage of  -casein by chymosin, a protease used in the fabrication of cheese.
-casein by chymosin, a protease used in the fabrication of cheese.
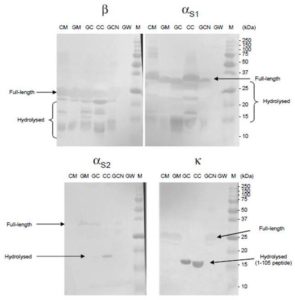
Figure 1: Western-Blot of Milk and Cheese Proteins Revealed with Caseins Specific Antibodies
Proteins from cow’s milk (CM), goat’s milk (GM), goat’s cheese (GC), cow’s cheese (CC), goat caseins (GCN) and goat whey (GW) were separated on 12% SDS-PAGE and blotted onto membranes. Membrane were incubated with monoclonal antibodies specific of each casein (αS1, αS2, β and  ). Bound antibodies were revealed by a colored method. M: molecular weight marker.
). Bound antibodies were revealed by a colored method. M: molecular weight marker.
Both patient #816 and #820 have relatively similar clinical profiles: anaphylaxis to G/SM and G/SM cheese (several accidents); less severe allergic reactions to CM cheese and tolerance of CM; both have high GM-specific IgE (>100kUA/L and 87 kUA/L respectively) and low CM-specific IgE (3.24 kUA/L and 1.9 kUA/L respectively) (Table 2). Western-blot (Fig. 2) confirmed the strong reactivity of both patients toward goat caseins in milk, cheese and casein fraction (correspondence between bands detected by patients IgE (Fig.2) and by antibodies specific for each casein (Fig.1)).
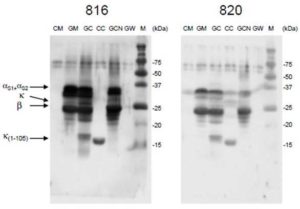
Figure 2: Western-Blot of Milk and Cheese Proteins Revealed with Serum from G/SMA Patients
Proteins from cow’s milk (CM), goat’s milk (GM), GM cheese (GC), CM cheese (CC), total goat caseins (GCN) and goat whey (GW) were separated on 12% SDS-PAGE and blotted onto membranes. Membrane were incubated with the sera of two patients (#816 and #820) and bound IgE revealed by a chemo-luminescence method. M: molecular weight marker.
No reactivity was found toward goat whey proteins (lines GW) (the two bands at Mr ~65000 and ~70000 are artifacts, they appear in every line even in case of totally negative serum). These results are consistent with previous observations (8). Patient 816 did not show any reactivity toward CM proteins and patient #820 showed a very low reactivity toward bovine αS-caseins and their degraded forms in CM and cow’s cheese. It is not possible to distinguish αS1- from αS2-casein but reactivity is most likely directed against αS1-casein which is much more abundant than αS2-casein. Surprisingly, both patients recognized strongly bovine  -casein 1-105 peptide from CM cheese whereas they did not show any reactivity toward full-length bovine
-casein 1-105 peptide from CM cheese whereas they did not show any reactivity toward full-length bovine  -casein (compare Fig.1 “” and Fig. 2). A similar reactivity was also observed against
-casein (compare Fig.1 “” and Fig. 2). A similar reactivity was also observed against  -casein 1-105 peptide from GM cheese. It is likely that the intermediate reactivity of these patients against CM cheese is mainly due to the strong and specific recognition of this 1-105
-casein 1-105 peptide from GM cheese. It is likely that the intermediate reactivity of these patients against CM cheese is mainly due to the strong and specific recognition of this 1-105  -casein fragment which is generated during cheese fabrication. The cleavage of
-casein fragment which is generated during cheese fabrication. The cleavage of  -casein by chymosin generates a strong immunogenic peptide and an immune reaction directed against an epitope which is not recognized in the full-length protein. Several hypothesis can be risen:1) the epitope is not accessible in the full-length protein; 2) a conformational epitope is generated because peptide 1-105 adopts a different conformation than full-length protein; 3) the epitope includes the C-terminal amino acid residues of 1-105 peptide and it is recognized only when the COOH of Phe105 is free (not included in peptide bond). This is a very uncommon situation in which proteolytic enzyme cleavage is able to generate a new strong immunogenic epitope.
-casein by chymosin generates a strong immunogenic peptide and an immune reaction directed against an epitope which is not recognized in the full-length protein. Several hypothesis can be risen:1) the epitope is not accessible in the full-length protein; 2) a conformational epitope is generated because peptide 1-105 adopts a different conformation than full-length protein; 3) the epitope includes the C-terminal amino acid residues of 1-105 peptide and it is recognized only when the COOH of Phe105 is free (not included in peptide bond). This is a very uncommon situation in which proteolytic enzyme cleavage is able to generate a new strong immunogenic epitope.
Acknowledgement
We thank Valérie Echasseriau for her excellent technical assistance.
Abbreviations
CMA: cow’s milk allergy; CM: cow’s milk; CG: goat’s milk; G/SM: goat/sheep’s milk; SM: sheep milk; G/SMA: goat/sheep’s milk allergy; CC: cow’s cheese; GC: goat’s cheese; GW: goat whey proteins; GCN: goat caseins; Mr: relative mass, SPT: skin prick test.
References
Ah-Leung, S., Bernard, H., Bidat, E., Paty, E., Rancé, F., Scheinmann, P. & Wal J.-M. (2006). “Allergy to Goat and Sheep Milk without Allergy to Cow’s Milk,” Allergy. 61 (11) 1358-65.
Publisher – Google Scholar
Attou, D., Caherec, A., Bensakhria, S., Dookna, P. & Faverge, B. (2005). “Allergie aux Laits de Chèvre et de Brebis sans Allergie Associée au Lait de Vache : Revue Générale à Propos d’une Observation à Rebondissements,” Revue Française d’Allergologie et d’Immunologie Clinique 45 601-07.
Publisher – Google Scholar
Dubuisson, C., La Vielle, S. & Martin, A. (2002). Allergies Alimentaires: État des Lieux et Propositions, AFSAA (ed.). Agence Française de Sécurité Sanitaire des Aliments (report).
Publisher – Google Scholar
Johansson, A., Lugand, D., Rolet-Répécaud, O. et al. (2009). “Epitope Characterization of a Supramolecular Protein Assembly with a Collection of Monoclonal Antibodies: The Case of Casein Micelle,” Molecular Immunology 46 1058-66.
Publisher – Google Scholar
Lamblin, C., Bourrier, T., Orlando, J. P., Sauvage, C. & Wallaert, B. (2001). “Allergie aux Laits de Chèvre et de Brebis sans Allergie Associée au Lait de Vache. Allergy to Goat’s or Sheep’s Milk without Allergy to Cow’s Milk,” Revue Française d’Allergologie et d’Immunologie Clinique 41 165-68.
Publisher – Google Scholar
Martins, P., Borrego, L. M., Pires, G., Pinto, P. L., Afonso, A. R. & Rosado-Pinto, J. (2005). “Sheep and Goat’s Milk Allergy — A Case Study,” Allergy 60 (1) 129-30.
Publisher – Google Scholar
Muñoz Martín, T., de la Hoz Caballer, B., Marañón Lizana, F., González Mendiola, R., Prieto Montaño, P. & Sánchez Cano, M. (2004). “Selective Allergy to Sheep’s and Goat’s Milk Proteins,” Allergologia et Immunopathologia (Madr) 32 (1) 39-42.
Publisher – Google Scholar
Restani, P., Gaiaschi, A., Plebani, A., Beretta, B., Cavagni, G., Fiocchi, A., Poiesi, C., Velonà , T., Ugazio, A. G. & Galli, C. L. (1999). “Cross-Reactivity between Milk Proteins from Different Animal Species,” Clinical & Experimental Allergy 29 (7) 997-1004.
Publisher – Google Scholar
Umpiérrez, A., Quirce, S., Marañón, F., Cuesta, J., García-Villamuza, Y., Lahoz, C. & Sastre, J. (1999). “Allergy to Goat and Sheep Cheese with Good Tolerance to Cow Cheese,” Clinical & Experimental Allergy 29 (8) 1064-68.
Publisher – Google Scholar
Viñas, M., Carnés, J., López-Matas, M. A., Hernández, N., Castillo, M. J. & Ibero, M. (2012). “Allergy to Goat and Sheep Cheese with Tolerance to Cow’s Milk and its Derivatives,” Allergologia et Immunopathologia (Madr) doi:pii: S0301-0546(12)00280-7.
Publisher – Google Scholar
Wüthrich, B. & Johansson, S. G. O. (1995). “Allergy to Cheese Produced from Sheep’s and Goat’s Milk but not to Cheese Produced from Cow’s Milk,” Journal of Allergy and Clinical Immunology
Publisher – Google Scholar
![]() -casein 1-105 peptide which is present in cow’s milk cheese but not in cow’s milk. This peptide is generated by cleavage of
-casein 1-105 peptide which is present in cow’s milk cheese but not in cow’s milk. This peptide is generated by cleavage of ![]() -casein by chymosin during cheese fabrication. The reactivity of patients with anaphylaxis to goat’s/sheep’s milk toward cow’s milk cheese is due to the presence of an epitope in bovine
-casein by chymosin during cheese fabrication. The reactivity of patients with anaphylaxis to goat’s/sheep’s milk toward cow’s milk cheese is due to the presence of an epitope in bovine ![]() -casein 1-105 peptide, epitope absent or not accessible in full-length bovine
-casein 1-105 peptide, epitope absent or not accessible in full-length bovine ![]() -casein.
-casein.