Introduction
Despite intensive investigations, many disorders of age-associated immune dysfunctions have yet to be described. Aging induces numerous immune system alterations, meaning immunosenescence. These changes cause refractory responses to vaccination or infection, reduction in previously established protective immunity and increased disease-related morbidity rate [1-6]. B cells have critical roles in the establishment and keeping of protective immunity, such as generation of protective immunoglobulins, antigen presentation, and appreciated regulatory activities [7]. Aging reduce class switch recombination and DNA recombination process which are needed for the manufacture of switched antibodies. This decrease which was noted in both mice [8] and humans [9,10], produces lower amount of immunoglobulin G (IgG) for an optimal recently generated antigen response.
Chronic and recurrent respiratory diseases, including rhinosinusitis, otitis media, and pneumonias have been correlated with antibody deficiencies. IgG and its subclasses deficiencies have been elucidated as a critical factor for chronic rhinosinusitis [11-13]. This study was conducted to evaluate the role of aging on IgG and its subclasses production in patients with chronic rhinosiusitis and to clarify the impact of these immunoglobulins in such chronic inflammatory diseases.
Methods and Material
All patients provided informed consent to participate in the study. This study was approved by the Ethics Committee of the Mazandaran University of Medical Sciences, Sari, IRAN.
The study population included 65 patients with chronic rhinosinusitis. Patients were recruited from the Ear, Nose, Throat section of the university hospital. All patients were selected according to criteria for chronic rhinosinusitis as described by the Sinus and Allergy Health Partnership [14].
In the opinion of investigators, exclusion criteria included the conditions that could affect the immunoglobulins levels such as malignancy (American Cancer Society guidelines for benign and malignant neoplasms were used for screening the patients before the initiation of the study), renal dysfunction, vascular diseases, malnutrition or patients receiving immunosuppressive medication, chemotherapy or radiation therapy or any other conditions that could make the subjects unsuitable for the research.
We used fasting serum samples of the volunteer patients. Venous samples were collected by venepuncture and let to clot naturally then serum separated.
Immunoglobulin G, IgG1, IgG2, IgG3 and IgG4 were measured by MININEPHTM HUMAN IgG KIT (The binding site Ltd., Birmingham, UK). The quantifications of serum IgG, IgG1, IgG2, IgG3 and IgG4 had been done by nephelometric procedure. For standard analysis; all assays were performed duplicate at the time of samples collection. For statistical analysis SPSS software, Version 16, Chicago, IL, USA was used to apply analysis of variance (ANOVA). P value of less than 0.05 was considered statistically significant.
Results
The study population consisted of 65 patients with chronic rhinosinusitis including 30 female and 35 male subjects. The demographic data of participants were summarized in table1. This series was divided to four groups including patients less than 20 year, 20 to 30 years, age of 30 to 40 years and patients more than 40 years old.
The youngest patients served as control group and other groups were compared with these subjects. There was significant change in IgG level of patients in category of 20 -30 year (p=0.02). In this relation, there was no significant differences in other age groups (table 2).
Table 1. Baseline Study Population Characteristics
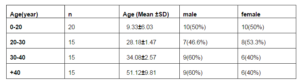
Table 2. Serum IgG and its Subclasses Concentrations. All Values are Presented as Mean ±SD, P Value of Less than 0.05 was Considered Statistically Significant
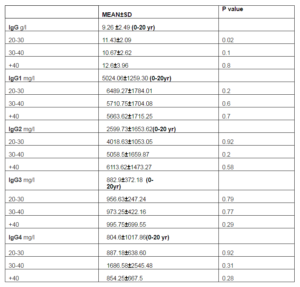
With comparison of patients with the oldest group (more than 40 years), there were significant changes in IgG2 and IgG3 in group of 20 — 30 years (p=0.04, P= 0.033, respectively). In this regard, significant difference was noted in IgG4 in group of 30-40 years (p=0.005).
Discussion
In the current research we examined the sera levels of immunoglobulin G and its subclasses in patients with chronic rhinosinusitis closely implicated with aging. Our study indicated there was significant change in IgG level of patients in age of 20 -30 years in comparison with youngest subjects. In comparison of patients with the oldest participants, there were significant differences in IgG2 and IgG3 in group of 20 — 30 years. Likewise, significant alteration was noted in IgG4 in group of 30-40 years. Not significantly, the amount of IgG2 and IgG3 increased with age.
B cell, T cell and other cells of the innate immune system have been showed in a suboptimal immune response in advanced age, but clarity considering the importance of intrinsic B cell contributions to this reduction and accurate biomarkers for B cell deficiencies with advance age have recently been indicated(15).Aging in mice imposes a decrease in manufacture of precursor B cells in the bone marrow [16-18], but the counts of mature splenic B cells is preserved, since to increased life-span [19].In consistent with mouse B cells, human peripheral B cell populations decrease with aging [20-22]. But there is a research [23] and a review article [24] indicating that memory B cell counts increase with age. It was revealed that in aged mice immune response to influenza has less IgG than that in young mice [25]. Despite these evidences, studies didn’t discuss about the effect of aging on IgG production and chronic rhinosinusitis. Different studies have indicated a high frequency of respiratory tract disorders in patients with IgG subclass deficiencies. The age at which each of the IgG subgroups arrive at adult levels differs and every age group in childhood has its own normal values [26,27]. Human IgG could be divided into four subclasses, IgG1, IgG2, IgG3, and IgG4. IgG1 is the major part of total IgG (66%), followed by IgG2 (24%), IgG3 (7%), and IgG4 (3%) (28,29).
In the present study, there was significant elevation in IgG level of patients in group of 20 to 30 years. IgG2 and IgG3 increased with aging but IgG, IgG1and IgG4 did not indicate the same pattern. Crisp HC and Quinn JM (30) examined the sera samples of the people aged 20-89 years old to analyze for quantitative IgG. They showed IgG levels were not significantly altered in an older population. Likewise, our study revealed that there was not significant alteration in most part of the population.
In conclusion, the defects discussed in the current article for IgG and its subclasses could lead to the discovery of procedures for improvement of humoral immune responses in the future. Recognizing the autonomous B cell biomarkers of aging that impose function involve reduced IgG class switch recombination and other associated factors (31). These findings show targets for intervention to improve the humoral immune system in aging.
Acknowledgments
The authors thank chronic rhinosinusitis patients, their siblings (family) and Milad Bahari for statistical assistance
References
1. Ben-Yehuda, A. & Weksler, M. E. (1992). “Immune Senescence: Mechanisms and Clinical Implications,” Cancer Investigation.10:525—531
Publisher – Google Scholar
2. Miller, R. A. (1996). “The Aging Immune System: Primer and Prospectus,” Science. 273:70—74.
Publisher – Google Scholar
3. Gruver, A. L., et al. (2007). “Immunosenescence of Ageing,” The Journal of Pathology. 211:144—156.
Publisher – Google Scholar
4. Kay, M. M., et al. (1979). “Age-Related Changes in the Immune System of Mice of Eight Medium and Long-Lived Strains and Hybrids. II. Short- and Long-Term Effects of Natural Infection with Parainfluenza Type 1 Virus (Sendai),”Mechanisms of Ageing and Development.11:347—362.
Publisher – Google Scholar
5. Ben-Yehuda, A. & Weksler, M. E. (1992). “Host Resistance and the Immune System,” Clinics in Geriatric Medicine. 8:701—711.
Publisher – Google Scholar
6. Ginaldi, L., et al. (2001). “Immunosenescence and Infectious Diseases,” Microbes and Infection. 3:851—857.
Publisher – Google Scholar
7. Frasca, D., et al. (2008). “Mechanisms for Decreased Function of B Cells in Aged Mice and Humans,” The Journal of Immunology. 180:2741—2746.
Publisher – Google Scholar
8. Frasca, D., Van der Put, E., Riley, R. L. & Blomberg, B. B. (2004). “Reduced Ig Class Switch in Aged Mice Correlates with Decreased E47 and Activation-Induced Cytidine Deaminase,” The Journal of Immunology.172:2155—2162.
Publisher – Google Scholar
9. Frasca, D., Diaz, A., Romero, M., Landin, A. M., Phillips, M., Lechner, S. C., Ryan, J. G. & Blomberg, B. B. (2010). “Intrinsic Defects in B Cell Response to Seasonal Influenza Vaccination in Elderly Humans,” Vaccine. 28:8077—8084.
Publisher – Google Scholar
10. Frasca, D., Landin, A. M., Lechner, S. C., Ryan, J. G., Schwartz, R., Riley, R. L. & Blomberg, B. B. (2008). “Aging Down-Regulates the Transcription Factor E2A, Activation-Induced Cytidine Deaminase, and Ig Class Switch in Human B Cells,” The Journal of Immunology.180:5283—5290.
Publisher – Google Scholar
11. Armenaka, M., Grizzanti, J. & Rosenstreich, D. L. (1994). “Serum Immunoglobulins and IgG Subclass Levels in Adults with Chronic Sinusitis: Evidence for Decreased IgG3 Levels,” Annals of Allergy 72: 507—14.
Publisher – Google Scholar
12. Shapiro, G. G., Virant, F. S., Furukawa, C. T., Pierson, W. E. & Bierman, C. W. (1991). “Immunologic Defects in Patients with Refractory Sinusitis,” Pediatrics 87: 311—6.
Publisher – Google Scholar
13. Chee, L., Graham, S. M., Carothers, D. G. & Ballas, Z. K. (2001). “Immune Dysfunction in Refractory Sinusitis in a Tertiary Care Setting,” The Laryngoscope 111: 233—5.
Publisher – Google Scholar
14. Meltzer, E. O., Hamilos, D. L., Hadley, J. A., Lanza, D. C., Marple, B. F. & Nicklas, R. A., et al. (2004). “Rhinosinusitis: Establishing Definitions for Clinical Research and Patient Care,” The Journal of Allergy and Clinical Immunology 114:155-212.
Publisher – Google Scholar
15. Frasca, D. & Blomberg, B. B. (2009). “Effects of Aging on B Cell Function,” Current Opinion in Immunology. 21(4): 425—430.
Publisher – Google Scholar
16. Sherwood, E. M., Blomberg, B. B., Xu, W., Warner, C. A. & Riley, R. L. (1998). ‘Senescent BALB/c Mice Exhibit Decreased Expression of Lambda 5 Surrogate Light Chains and Reduced Development within the Pre-B Cell Compartment,’ The Journal of Immunology.161:4472—4475.
17. Miller, J. P. & Allman, D. (2003). “The Decline in B Lymphopoiesis in Aged Mice Reflects Loss of Very Early B-Lineage Precursors,” The Journal of Immunology.171:2326—2330.
Publisher – Google Scholar
18. Alter-Wolf, S., Blomberg, B. B. & Riley, R. L. (2009). “Deviation of the B Cell Pathway in Senescent Mice is Associated with Reduced Surrogate Light Chain Expression and Altered Immature B Cell Generation, Phenotype, and Light Chain Expression,” The Journal of Immunology.182:138—147.
Publisher – Google Scholar
19. Miller, J. P. & Cancro, M. P. (2007). “B Cells and Aging: Balancing the Homeostatic Equation,” Experimental Gerontology. 42:396—399. B Cells from Aged Mice Survive Better in Response to BlyS, Helping to Explain the Maintenance of Peripheral B Cells although Being Derived from Fewer Precursors.
Publisher – Google Scholar
20. Franceschi, C., Monti, D., Sansoni, P. & Cossarizza, A. (1995). “The Immunology of Exceptional Individuals: The Lesson of Centenarians,” Immunology Today.16:12—16.
Publisher – Google Scholar
21. Shi, Y., Yamazaki, T., Okubo, Y., Uehara, Y., Sugane, K. & Agematsu, K. (2005). “Regulation of Aged Humoral Immune Defense against Pneumococcal Bacteria by IgM Memory B Cell,” The Journal of Immunology.175:3262—3267.
Publisher – Google Scholar
22. Frasca, D., Landin, A. M., Lechner, S. C., Ryan, J. G., Schwartz, R., Riley, R. L. & Blomberg, B. B. (2008). “Aging Down-Regulates the Transcription Factor E2A, Activation-Induced Cytidine Deaminase, and Ig Class Switch in Human B Cells,” The Journal of Immunology.180:5283—5290. Aged human B cells have less total B cells, less class switch memory B cells, and show less E47, AID, and CSR when stimulated in vitro with anti-CD40 plus IL-4.
Publisher – Google Scholar
23. Colonna-Romano, G., Bulati, M., Aquino, A., Scialabba, G., Candore, G., Lio, D., Motta, M., Malaguarnera, M. & Caruso, C. (2003). “B Cells in the Aged: CD27, CD5, and CD40 Expression,” Mechanisms of Ageing and Development.124:389—393.
Publisher – Google Scholar
24. Siegrist, C. A. & Aspinall, R. (2009). “B-Cell Responses to Vaccination at the Extremes of Age,” Nature Reviews Immunology. 9:185—194.
Publisher – Google Scholar
25. Han, S., Yang, K., Ozen, Z., Peng, W., Marinova, E., Kelsoe, G. & Zheng, B. (2003). “Enhanced Differentiation of Splenic Plasma Cells but Diminished Long-Lived High-Affinity Bone Marrow Plasma Cells in Aged Mice,” The Journal of Immunology.170:1267—1273.
Publisher – Google Scholar
26. Schur, P. H., Rosen, F. & Norman, M. E. (1979). “Immunoglobulin Subclasses in Normal Children,” Pediatric Research 13: 181—3.
Publisher – Google Scholar
27. Aksu, G., Kutukculer, N., Ferah, G., Koturoglu, G. & Kurugol, Z. (2006). ‘Serum Immunoglobulin (IgG, A, M) and IgG Subclass Concentrations in Healthy Children: A Study Using Nephelometric Technique, Turk J Pediatr 48: 19—24.
28. Lawton, A. R. (1999). “IgG Subclass Deficiency and the Day-Care Generation,” Pediatric Infectious Disease Journal18: 462—6.
Publisher – Google Scholar
29. Pan, Q. & Hammarstrom, L. (2000). “Molecular Basis of IgG Subclass Deficiency,” Immunological Reviews: 178: 99—110.
Publisher – Google Scholar
30. Crisp, H. C. & Quinn, J. M. (2009). “Quantitative Immunoglobulins in Adulthood,” Allergy and Asthma Proceedings. 30(6):649-54.
Publisher – Google Scholar
31. Frasca, D. & Blomberg, B. B. (2011). “Aging Impairs Murine B Cell Differentiation and Function in Primary and Secondary Lymphoid Tissues,” Aging and Disease. 2(5): 361—373
Publisher – Google Scholar