Introduction
The diagnosis of large vessel vasculitis (LVV), as Giant cell arteritis (GCA) and Takayasu’s arteritis (TA), in clinical practice is often a difficult task, and it usually requires time and financial resources in order to define the characteristics of disease in patients with nonspecific symptoms and elevated inflammatory markers. In particular, two subsets of patients are a diagnostic challenge: 1) patients with fevers of unknown origin (FUOs), who can be classified into 4 main clinical categories: infectious, malignant, rheumatic-inflammatory and miscellaneous disorders (Meller et al. 2007, and Roth et al. 2003); 2) patients with increased inflammatory markers and systemic signs/symptoms in the absence of infections or tumors, (Gaeta et al. 2006).
Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitis (2013), (Jennette et al. 2012), classified vasculitis by:
1) Vessel diameter
2) Presence of granulomatous lesions
3) Presence of antineutrophil cytoplasmic antibodies (ANCA).
Standard diagnostic procedures include laboratory tests, biopsy, angiography, ultrasound and magnetic resonance angiography (Fuchs 2012), although these conventional diagnostic methods are not adequate to make a definitive diagnosis in approximately 50% of patients in this small group.
In studies by Mukhtyar (2009) and Basu (2012), laboratory tests, including erythrocyte sedimentation rate (ESR), C-reactive protein (PCR) and ANCA antibodies, were found to be nonspecific for large vessel vasculitis diagnosis.
Conventional imaging techniques such as Computed Tomography — (CT), angio-Computed Tomography (angio-CT), Magnetic Resonance (MR) and/or angio – Magnetic Resonance (angio-MR), ultrasound, and biopsy can be invasive, operator dependent, and often biased during disease duration and therapy. In particular MR takes relatively long time, can study only one region at time and cannot be performed in patients with pace maker. Angio-CT cannot be performed in patients with kidney disease because of the contrast.
Positron emission tomography-computed tomography (PET/CT), instead, is a noninvasive, operator independent, metabolic imaging modality based on the regional distribution of the glucose analogue (fluorine-18 fluorodeoxyglucose — 18F-FDG) (Fuchs 2012) and can be performed in all kinds of patients. In retrospective studies by Cao (2012), Qiu (2012), Watts (2009), Tezuka (2012), Talarico (2012) and Pipitone (2008) the performance of FDG PET/CT to diagnose vasculitis, rheumatic diseases, inflammatory diseases, and peritoneal fibrosis was evaluated usually enrolling a low amount of patients.
Sheng (2011), Cao (2012) and Qiu (2012) suggested that for the correct diagnosis with PET the addition of conventional imaging techniques may generally offer improved diagnostic accuracy compared with current standards of practice.
The aim of this study was the diagnostic accuracy of PET in patients with fever of unknown origin (FUO) and in patients with suspected and/or proved large vessel vasculitis treated with and without immunosuppressive drugs, and the possibility of the use of PET in these diseases’ monitoring and treatment.
Patients and methods
Patients
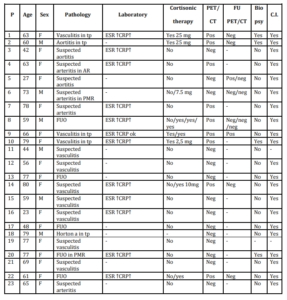
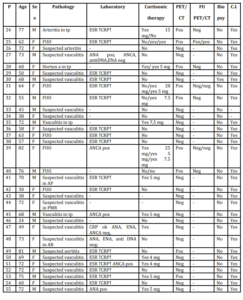
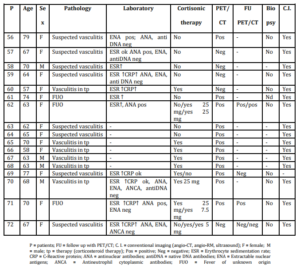
72 patients (49 females, 23 males; mean age 63 y, range 23-82), who were referred to our interdisciplinary centre in the Department of Rheumatology, were prospectively examined with PET imaging between February 2010 and April 2013; 42 with suspected large vessel vasculitis (LVV) 15 with (LVV) during steroid therapy (over 5 mg of prednisone/day) and 15 with fever of unknown origin (FUO). Follow-up scans were performed in 19 patients. Diagnoses were based on the American College of Rheumatology (ACR) classification criteria, laboratory tests, conventional imaging (angio-MRI and/or angio-CT, and/or ultrasound), on temporal artery biopsy in some patients and the exclusion of other diagnoses (Tab 1).
Table 1: Patient studied with PET/CT
According to validated diagnostic criteria for vasculitis and for FUO, these are the main criteria we used to have our patients undergo PET imaging (13, 4), (Tab. 2 and Tab. 3).
Table 2: Vasculitis diagnostic main criteria
Table 3: FUO main diagnostic criteria (PET should be performed before bone marrow biopsy).
The study’s endpoint was the diagnostic accuracy of PET for large vessel vasculitis (LVV) patients treated with and without immunosuppressive drugs, and the possibility of the use of PET for disease monitoring and treatment.
All scans were acquired after a 6hour fast, using an integrated PET/CT camera (PHILIPS GEMINI TF), equipped with a full-ring dedicated PET scanner and a sixteen slice CT scanner. The serum glucose level was measured before 18F-FDG administration in all patients and was below 120 mg/dl (Glucometer Breeze 2, Bayer Diagnostics). PET scans were performed 60 min after intravenous injection using a venous line of 3,7 MBq/Kg body weight of 18F-FDG. Low dose CT (max 120 Kv, 100mAs) and PET scans were performed from the base of the skull to the mid thigh. We performed whole-body scans using 3D acquisition with 7-8 contiguous bed positions (2min/bed). Iterative ordered subset expectation maximization (OSEM) reconstruction algorithm was used to obtain 512×512 format trans-axial slices of 4-5 mm thickness; CT attenuation correction was performed. Philips Imaging software was used to view and analyze the reconstructed images in PET-CT fusion modality.
PET Imaging and Statistical Analysis
All scans were assessed by a panel of board certified nuclear medicine specialists.
PET scans were analysed visually in a descriptive manner, as well as by a quantitative computed method.
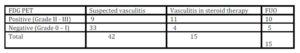
First visual analysis was performed. Thereafter, image readings were divided into three groups: normal vessel uptake, abnormal uptake in vessels with atherosclerotic plaque, and abnormal uptake in vessel without atherosclerotic plaque(Fig 1a, 1b, 1c).
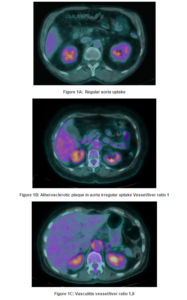
Figure 1: FDG uptake in normal aorta wall (FIG 1A); FDG uptake in atherosclerotic wall of aorta (Fig 1 B): calcific plaque were considered as false positives; FDG uptake in vasculitis (Fig 1 C).
For quantitative evaluation, region of interest (ROI) was placed over nine defined vascular areas (ascending thoracic aorta, descending thoracic aorta, abdominal aorta, right and left subclavian arteries, right and left external carotid arteries, right and left common iliac arteries) and right hepatic lobe.
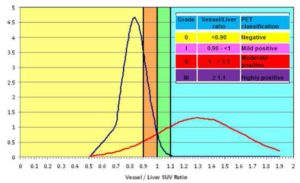
Using regions of interest SUVmax vessel to liver ratio was calculated and rated into 4 Grades. The means and standard deviations were calculated for orderly vessel/liver uptake ratio distributions, divided into two groups: negative (<1.0) and highly positive (≥ 1.0). For each group, the normal distribution was computed. Statistical analysis calculated different intervals (vessel/liver ratio < 0.9 was considered grade 0; vessel/liver ratio between 0.9 and 1 was considered grade I; vessel/liver ratio between 1 and1.1 was considered grade II; vessel/liver ratio ≥ 1.1 was considered grade III). Grade II and III were considered pathological.
Sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of FDG PET/CT were calculated using clinical criteria and conventional imaging as the reference standard.
Results
The reference point for highly positive cases (≥ 1.1, blue area) was identified by the overlap of the two normal distributions. The mildly and moderately positive grades (mild: range 0.9- <1.0, orange area, and moderate: range 1.0- <1.1, green area) were defined by the presence of the positive distribution tail subtending the negative normal distribution(Fig2).
Using a semiquantitative analysis based on SUV max vessel to liver ratio, PET scans were considered positive for large vessel vasculitis in 30/72 (42%) patients, and negative in 42/72 (58%) patients. In 42 patients with suspected LVV: 9 (21%) were positive on PET scan, and 33 (79%) were negative; in 15 patients with LVV during steroid therapy: 11 (73%) were positive on PET scan, and 4 (27%) negative; in 15 patients with fever of unknown origin (FUO): 10 (67%) were PET positive and 5 (33%) negative (Table 4, Fig 3).
Table 4: FDG PET/CT results
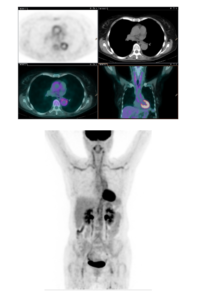
Considering clinical criteria and conventional imaging, PET had an overall sensitivity of 87% [95% confidence interval (CI) 79-95%], a specificity of 95% (95% CI 90-100%), a positive predictive value of 93% (95% CI 87-99%) and a negative predictive value of 91% (95% CI 84-97%). The diagnostic accuracy of PET was 92% (95% CI 85-98%).
As reported by Fuchs et al.(2012), specificity and sensitivity of PET can be lower (64.5%) in patients on immunosuppressive or steroid therapy than in untreated patients. In our analysis we have considered all patients on steroid therapy as treated (over 5 mg of prednisone/day) because statistically significant differences were found.
We also utilized PET to detect therapy response in 19 patients affected by LVV. These patients were evaluated at baseline and at a different time point of follow up (when clinical relapse was evident or after steroid therapy reduction or withdrawal). Even if the number of patients was low for statistical analysis we could see a good correlation between positivity of PET and clinical symptoms.
Discussion
The diagnosis of large vessel vasculitis (LVV) is often difficult, and it usually requires time, money and discomfort for the patient. The diagnosis of small vessel vasculitis is still histological.
As reported by Hooisma (2012), the diagnostic standard method for LVV, especially in giant cell arteritis (GCA), is a temporal artery biopsy, but the test results can be false negative in 15-70%, which may delay the diagnosis; in Takayasu’s arteritis (TA), which affects the aorta and its main branches, as well as the coronary and pulmonary arteries, routine histological examination is not suitable.
Several methods such as Color Doppler Sonography (CDS), Magnetic Resonance (MR) and Computed Tomography (CT) have been proposed to evaluate LVV. As reported by Pipitone et al. (2008) these tools (CDS, MRI, CT) revealed their usefulness in detecting early vasculitic lesions (mainly presented as an alteration of vessel lumen), while only angiography was able to detect delayed effects of LVV (e.g. aneurism or vascular stenosis).
Besson and coworkers (2011) reported in their meta-analysis that diagnostic performance of FDG-PET in giant cell arteritis provided sensitivity and specificity of 80% and 89%, respectively.
In their study, Ergul (2011) and Hooisma (2012) highlighted the utility of PET in early diagnosis of LVV and in discovery of occult inflammatory or neoplastic disorders.
In our study, the sensitivity and specificity of PET are high, with lower risk to patients with respect to other diagnostic methods (such as angiography or CT/MR angiography). As previously reported by Fuchs et al., 18F-FDG PET/CT increases the overall diagnostic accuracy and has an impact on the medical management in a significant proportion of patients, but it is unlikely to replace biopsy procedures in the near future. PET-CT without contrast allows evaluating the extension of vascular involvement; however, it cannot accurately analyze the vascular wall. The intensity of vascular inflammation, moreover, can help in the differential diagnosis and it can be a useful tool for monitoring response to therapy.
As described by Kumar et al (2012)., the correlation between the inflammatory status and FDG uptake also allows to use PET in follow up, for evaluating response to LVV therapy, and to detect possible flares before complications arise.
In a prospective study by Blockmans et al. (2006) involving 35 patients (CAO) with GCA, a significant decrease in vascular FDG uptake at 3 months indicated a potential future response to treatment.
As with previous reports, we confirm the lower diagnostic accuracy in patients on immunosuppressive therapy. We chose a cut off of 5 mg/day of prednisone for a week because this dose does not seem to interfere with SUV, but it is often impractical to not treat a severe vasculitis before obtaining a PET scan.
Even our data, in agreement with what is reported in the literature, do not allow to express an opinion on a possible involvement of cerebral vessels given the high uptake of FDG in brain tissue.
Qui Lin et al. (2012) reviewed the literature on the use of PET in the diagnosis of FUO, and it was found that PET’s mean sensitivity was 80-90% and specificity was 88-90%. FDG PET scans were examined in various cases. A definitive diagnosis was made in 50% of cases and 20% of cases were related to inflammatory processes of the great vessels. Therefore, Yang et al. (2012) confirmed the superiority of PET among the various diagnostic techniques used in the diagnosis of FUO, with an excellent cost benefit profile, the possibility of reducing the number of surveys and the duration of hospitalization.
In our study, the use of PET in patients with FUO has allowed us to make a diagnosis in 80% of cases, of which 70% was a vasculitis.
Conclusion
18F-FDG PET/CT (PET) is a sensitive and specific imaging tool for large vessel vasculitis (LVV) and FUO; it increases the overall diagnostic accuracy and has an impact on the clinical management in a significant proportion of patients for several reasons. FUO might be caused by a vasculitis. PET is a one-shot diagnostic method (cost saving) as reported by Becerra et al. (2012). Its diagnostic accuracy precludes the need for arterial biopsy, and instead an easy semi-quantitative analysis can be used for the right diagnosis of vasculitis. Finally, PET is a good tool for the diagnosis of LVV, but also for therapy monitoring and follow up.
However, since this method still encounters some resistance to be used as the gold standard in the diagnostic guidelines of FUO, further studies are needed to confirm the specificity, accuracy and sensitivity as well as the cost benefit analysis in the diagnosis of vasculitis.
Disclosures
None
References
1. Basu, N., Watts, R., Bajema, I., Baslund, B., Bley, T., Boers, M., et al. (2010), ‘EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis,’ Annals of the Rheumatic Diseases, 69,1744-50.
Publisher – Google Scholar
2. Becerra Nakayo, EM., García Vicente, AM., Soriano Castrejón, AM., Mendoza Narváez, JA., Talavera Rubio, MP., Poblete García, VM. and Cordero García, JM. (2012), ‘Analysis of cost-effectiveness in the diagnosis of fever of unknown origin and the role of (18)F-FDG PET-CT: a proposal of diagnostic algorithm,’ Revista Espaňola de Medicina Nuclear e Imagen Molecular, Jul-Aug, 31(4),178-86.
Google Scholar
3. Besson, FL., Parienti, JJ., Bienvenu, B., Prior, JO., Costo, S., Bouvard, G. and Agostini, D. (2011), ‘ Diangnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systemic review and meta-analysis,’ European Journal of Nuclear Medicine and Molecular Imaging, Sept. 38(9), 1764-72.
Publisher – Google Scholar
4. Blockmans, D., de Ceuninck, L., Vanderschueren, S., et al. (2006), ‘Repetitive 18F-fluorodeoxyglucose positronemission tomography in giant cell arteritis: a prospective study of 35 patients,’ Arthritis and Rheumatology, 55, 131—7.
Publisher – Google Scholar
5. Cao, Q. and Chen, W. (2012), ‘ FDG PET Imaging of Large-Vessel Vasculitis,’ PET Clinics, 7, 227—232.
6. Cheng, Y., Lv, N., Wang, Z., Chen, B. and Dang, A. (2013), ‘18FDG PET in assessing disease activity in takayasu arteritis: a meta-analysis,’ Clinical and Experimental Rheumatology, 31(1 Suppl 75), S22-7.
Google Scholar
7. Chow, A. and Robinson, JL. (2011), ‘Fever of unknown origin in children: a systematic review,’ World Journal of Pediatrics, Feb, 7(1), 5-10.
Publisher – Google Scholar
8. Ergül, N. and Cermik, TF. (2011), ‘FDG-PET or PET/CT in fever of unknown origin: the diagnostic role of underlying primary disease,’ International Journal of Molecular Imaging, 2011, 318051.
Publisher – Google Scholar
9. Fuchs, M., Briel, M., Daikeler, T., Walker, UA., Rasch, H., Berg, S., Ng, Q., Raatz, H., Jayne, D., Kötter, I., Blockmans, D., Cid, MC., Prieto-González, S., Lamprecht, P., Salvarani, C., Karageorgaki, Z., Watts, R., Luqmani, R., Müller-Brand, J., Tyndall, A. and Walter, MA. (2012), ‘The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis,’ European Journal of Nuclear Medicine and Molecular Imaging, 39, 344—35.
Publisher – Google Scholar
10. Gaeta, GB., Fusco, FM. and Nardiello, S. (2006), ‘Fever of unknown origin: a systematic review of the literature for 1995—2004,’ Nuclear Medicine Communications, 27(3), 205—11.
Publisher – Google Scholar
11. Hooisma, GA., Balink, H., Houtman, PM., Slart, RHJA. and Lensen, KDF. (2012), ‘Parameters related to a positive test result for FDG PET(/CT) for large vessel vasculitis: a multicenter retrospective study,’ Clinical Rheumatology, 31, 861—871.
Publisher – Google Scholar
12. Jennette, C., Falk, RJ., Bacon, PA., Basu, N., Cid, MC., Ferrario, F., Flores-Suarez, LF., Gross, WL., Guillevin, L., et al (2013), ‘2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides,’ Arthritis and Rheumatology, 65(65), 1—11.
Publisher – Google Scholar
13. Kumar, R., Karunanithi, S., Zhuang, H., Alavi, A. (2012), ‘Assessment of Therapy Response by FDG PET in Infection and Inflammation,’ PET Clinics, 7, 233—243.
Publisher – Google Scholar
14. Meller, J., Sahlmann, CO. and Scheel, AK. (2007), ‘18F-FDG PET and PET/CT in fever of unknown origin,’ Journal of Nuclear Medicine, 48(1), 35—45.
Google Scholar
15. Mukhtyar, C., Guillevin, L., Cid, MC., Dasgupta, B., de Groot, K., Gross, W., et al. (2009), ‘EULAR recommendations for the management of large vessel vasculitis,’ Annals of the Rheumatic Diseases, 68, 318—23.
Google Scholar
16. Papathanasiou, ND., Du, Y., Menezes, LJ., Almuhaideb, A., Shastry, M., Beynon, H. and Bomanji, JB. (2012). ‘18F-Fludeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: Diagnostic performance and correlation with clinical and laboratory parameters,’ British Journal of Radiology, 85, 1014 (e188-e194).
Google Scholar
17. Pipitone, N., Versari, A. and Salvarani, C. (2008), ‘Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update,’ Rheumatology, 47, 403—408.
Publisher – Google Scholar
18. Qiu, L. and Chen, Y. (2012), ‘The role of 18F-FDG PET or PET/CT in the detection of fever of unknown origin,’European Journal of Radiology, 81, 3524— 3529.
Publisher – Google Scholar
19. Roth, AR. and Basello, GM. (2003), ‘Approach to the adult patient with fever of unknown origin,’ American Family Physician, 68(11), 2223—8.
Google Scholar
20. Schmidt, WA. (2013), ‘Imaging in vasculitis,’ Best practice & research clinical rheumatology, 27, 107-118.
Publisher – Google Scholar
21. Sheng, JF., Sheng, ZK., Shen, XM., Bi, S., Li, JJ., Sheng, GP., Yu, HY., Huang, HJ., Liu, J., Xiang, DR., Dong, MJ., Zhao, K. and Li, LJ. (2011), ‘Diagnostic value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with fever of unknown origin,’ European Journal of Internal Medicine, 22(1), 112—6.
Publisher – Google Scholar
22. Talarico, R., Baldini, C., Della Rossa, A., Stagnaro, C., Ferrari, C., Luciano, N. and Bombardieri, S. (2012), ‘Large- and small-vessel vasculitis: a critical digest of the 2010-2011 literature,’ Clinical and Experimental Rheumatology, 30(Suppl. 70), S130-S138.
23. Tezuka, D., Go Haraguchi, G., Ishihara, T., Ohigashi, H., Inagaki, H., Suzuki, J., Hirao, K. and Isobe, M. (2012),‘Role of FDG PET-CT in Takayasu Arteritis Sensitive Detection of Recurrences,’ Journal of the American College of Cardiology, 5(4), 422-9.
Publisher – Google Scholar
24. Watts, R., Al-Taiar, A., Mooney, J., et al. (2009), ‘The epidemiology of Takayasu arteritis in the UK,’Rheumatology(Oxford), 48(8), 1008—11.
Publisher – Google Scholar
25. Yang, J., Zhuang, H. and Servaes, S. (2012), ‘Fever of Unknown Origin: The Roles of FDG PET or PET/CT,’ PET Clinics, 7, 181—189.