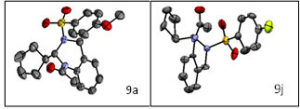
Fig2: Single X-ray crystal structures of compounds 9a and 9j
Conclusion
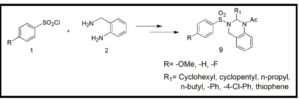
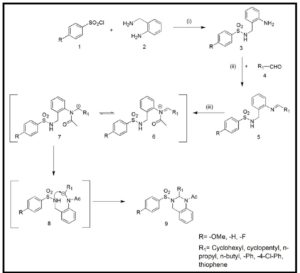
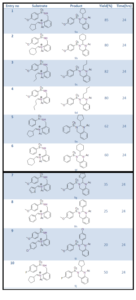
We have successfully synthesized 10 N1-acyl-N2-sulfono Quinazoline derivatives via N-acylinium intermediate and also achieved the cyclization with electron deficient sulfonamide as nucleophile. So, this general strategy for pursuing highly active electrophiles to facilitate the intramolecular nucleophilic attack becomes a basis for synthesizing biologically active more functionalized quinazoline derivatives. Higher yield of the cyclized product was observed in case of activated aromatic ring. Since these N1-acyl-N2-sulfono Quinazolines obtained as racemic mixture, the streoselective version of this cyclization is our next target. The library of these new heterocyclic scaffold and the compounds therefore may act as good inhibitors of different enzymes or proteins. Biological studies of these molecules are going on in the present time in our laboratory, and the results will be published in the future.
Experimental Section
General Procedure for the Final Cyclization
To a stirred solution of schiff base (5) (1 mmole) dissolved in THF or diethylether (as per requirement) at 00 C, 2,6-leutidine (1 mmole) was added followed by acetyl chloride (1mmole) addition under nitrogen atmosphere (scheme 2). The reaction was continued for 24hrs. The solvent was evaporated to concentrate the reaction mass and the residue obtained was purified by column chromatography (hexane/ethyl acetate) to afford pure cyclized product.
1-(2-Cyclopentyl-3-(4-methoxy-benzenesulfonyl)-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9a): White solid, Rf  0.40 (petroleum ether, ethyl acetate 3:2), Melting point 1550 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 1.327-1.490 (4H, m), 1.641-1.710 (4H, m), 1.833 (3H, s), 1.918 (1H, m), 3.807 (3H, s), 4.525 (1H, d, J = 17.4), 4.780 (1H, d, J = 18), 6.325 (1H, d, J = 10.2), 6.873-6.912 (3H, m), 7.146-7.207 (3H, m), 7.727 (2H, d, J = 7.2); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.61, 25.08, 25.19, 27.83, 28.84, 41.30, 43.49, 55.55, 66.46, 114.07, 114.54, 124.45, 125.18, 125.50, 126.43, 127.18, 129.33, 129.88, 130.53, 135.18, 162.89, 169.01; HRMS (EI+): m/z Calcd for C22H26N2O4S (M)+ 414.1613, Found: m/z 414.16132. IR (in cm-1) 565, 615.56, 671.30, 748.91, 804.99, 835.42, 962.61, 1020.74, 1091.86, 1160.93, 1264.53, 1322.65, 1339.75, 1378.73, 1499.43, 1596.53, 1654.66, 2867.10, 2962.84, 3008.32, 3445.52.
0.40 (petroleum ether, ethyl acetate 3:2), Melting point 1550 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 1.327-1.490 (4H, m), 1.641-1.710 (4H, m), 1.833 (3H, s), 1.918 (1H, m), 3.807 (3H, s), 4.525 (1H, d, J = 17.4), 4.780 (1H, d, J = 18), 6.325 (1H, d, J = 10.2), 6.873-6.912 (3H, m), 7.146-7.207 (3H, m), 7.727 (2H, d, J = 7.2); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.61, 25.08, 25.19, 27.83, 28.84, 41.30, 43.49, 55.55, 66.46, 114.07, 114.54, 124.45, 125.18, 125.50, 126.43, 127.18, 129.33, 129.88, 130.53, 135.18, 162.89, 169.01; HRMS (EI+): m/z Calcd for C22H26N2O4S (M)+ 414.1613, Found: m/z 414.16132. IR (in cm-1) 565, 615.56, 671.30, 748.91, 804.99, 835.42, 962.61, 1020.74, 1091.86, 1160.93, 1264.53, 1322.65, 1339.75, 1378.73, 1499.43, 1596.53, 1654.66, 2867.10, 2962.84, 3008.32, 3445.52.
1-(2-Cyclohexyl-3-(4-methoxy-benzenesulfonyl)-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9b): White solid, Rf  0.50 (petroleum ether, ethylacetate 3:2), Melting point 1200 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.86-0.94 (1H, m), 1.05-1.17 (3H, m), 1.38-1.41 (1H, m), 1.55 (1H, d, J = 9.2), 1.65 (3H, s), 1.77 (1H, d, J = 18), 1.84 (3H, s), 1.93-1.94 (1H, m), 3.80 (3H, s), 4.46 (1H, d, J = 18), 4.76 (1H, d, J = 18), 6.27-6.28 (1H, m), 6.86-6.90 (3H, m) 7.14-7.20 (3H, m), 7.71 (2H, d, J = 6); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.62, 25.46, 25.91, 27.99, 28.88, 29.67, 37.96, 43.29, 55.55, 66.89, 114.08, 114.39, 124.43, 125.05, 125.52, 126.48, 127.16, 129.35, 129.84, 130.63, 134.93, 162.88, 169.27; HRMS (EI+): m/z Calcd for C23H28N2O4S (M)+ 428.17698, Found: m/z 428.17697. IR (in cm-1) 561.54, 617.27, 671.30, 746.86, 804.99, 833.02, 964.66, 1020.74, 1039.89, 1147.93, 1165.03, 1264.18, 1322.65, 1378.39, 1462.50, 1447.45, 1499.43, 1585.59, 1596.19, 1654.66, 2848.75, 2923.52, 3435.03.
0.50 (petroleum ether, ethylacetate 3:2), Melting point 1200 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.86-0.94 (1H, m), 1.05-1.17 (3H, m), 1.38-1.41 (1H, m), 1.55 (1H, d, J = 9.2), 1.65 (3H, s), 1.77 (1H, d, J = 18), 1.84 (3H, s), 1.93-1.94 (1H, m), 3.80 (3H, s), 4.46 (1H, d, J = 18), 4.76 (1H, d, J = 18), 6.27-6.28 (1H, m), 6.86-6.90 (3H, m) 7.14-7.20 (3H, m), 7.71 (2H, d, J = 6); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.62, 25.46, 25.91, 27.99, 28.88, 29.67, 37.96, 43.29, 55.55, 66.89, 114.08, 114.39, 124.43, 125.05, 125.52, 126.48, 127.16, 129.35, 129.84, 130.63, 134.93, 162.88, 169.27; HRMS (EI+): m/z Calcd for C23H28N2O4S (M)+ 428.17698, Found: m/z 428.17697. IR (in cm-1) 561.54, 617.27, 671.30, 746.86, 804.99, 833.02, 964.66, 1020.74, 1039.89, 1147.93, 1165.03, 1264.18, 1322.65, 1378.39, 1462.50, 1447.45, 1499.43, 1585.59, 1596.19, 1654.66, 2848.75, 2923.52, 3435.03.
1-[3-(4-methoxy-benzenesulfonyl)-2-propyl-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9c): White solid, Rf  0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1300 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.86-0.89 (3H, m), 1.39-1.44 (2H, m), 1.59 (3H, s), 1.87-1.88 (2H, m), 3.81 (3H, s), 4.49 (1H, d, J = 12), 4.63 (1H, d, J = 18), 6.63-6.66 (1H, m), 6.89 (2H, d, J = 6), 6.93-6.94 (1H, m), 7.15-7.16 (2H, m), 7.21 (1H, t, J = 6), 7.74 (2H, d, J = 12); 13C NMR (150 MHz, CDCl3): δ (in ppm) 13.42, 18.18, 22.66, 34.78, 43.40, 55.56, 62.68, 113.51, 114.16, 124.65, 125.42, 125.67, 126.36, 126.58, 127.33, 129.45, 129.79, 130.27, 162.99, 168.91; HRMS (EI+): m/z Calcd for C20H24N2O4S (M)+ 388.14568, Found: m/z 388.14554. IR (in cm-1) 557.10, 615.22, 632.66, 673.35, 722.93, 753.36, 759.85, 802.94, 835.08, 928.08, 958.17, 1027.23, 1037.83, 1089.46, 1113.40, 1147.93, 1158.53, 1262.13, 1309.66, 1328.81, 1372.23, 1460.45, 1497.03, 1585.59, 1596.19, 1652.26, 1663.20, 2866.08, 2923.52, 2975.49, 3435.03.
0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1300 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.86-0.89 (3H, m), 1.39-1.44 (2H, m), 1.59 (3H, s), 1.87-1.88 (2H, m), 3.81 (3H, s), 4.49 (1H, d, J = 12), 4.63 (1H, d, J = 18), 6.63-6.66 (1H, m), 6.89 (2H, d, J = 6), 6.93-6.94 (1H, m), 7.15-7.16 (2H, m), 7.21 (1H, t, J = 6), 7.74 (2H, d, J = 12); 13C NMR (150 MHz, CDCl3): δ (in ppm) 13.42, 18.18, 22.66, 34.78, 43.40, 55.56, 62.68, 113.51, 114.16, 124.65, 125.42, 125.67, 126.36, 126.58, 127.33, 129.45, 129.79, 130.27, 162.99, 168.91; HRMS (EI+): m/z Calcd for C20H24N2O4S (M)+ 388.14568, Found: m/z 388.14554. IR (in cm-1) 557.10, 615.22, 632.66, 673.35, 722.93, 753.36, 759.85, 802.94, 835.08, 928.08, 958.17, 1027.23, 1037.83, 1089.46, 1113.40, 1147.93, 1158.53, 1262.13, 1309.66, 1328.81, 1372.23, 1460.45, 1497.03, 1585.59, 1596.19, 1652.26, 1663.20, 2866.08, 2923.52, 2975.49, 3435.03.
1-(3-benzenesulfonyl-2-cyclopentyl-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9e): White solid, Rf  0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1100 C, 1H NMR (600 MHz,CDCl3): δ (in ppm) 1.21-1.26 (1H, m), 1.32-1.38 (2H, m), 1.42-1.45 (1H, m), 1.61-1.62 (1H, m), 1.65-1.66 (3H, m), 1.70 (3H, s), 1.86-1.87 (1H, m), 4.46 (1H, d, J = 18), 4.75 (1H, d, J = 18), 6.28 (1H, d, J = 6), 6.82 (1H, d, J = 12), 7.08-7.13 (3H, m), 7.33-7.36 (2H, m), 7.41-7.44 (1H, m), 7.72 (2H, d, J = 6); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.66, 25.09, 25. 19, 27.82, 28.82, 41.21, 43.61, 66.48, 124.44, 124.92, 125.54, 126.41, 127.22, 127.69, 129.00, 132.59, 138.97, 169.06; HRMS (EI+): m/z Calcd for C21H24N2O3S(M)+ 384.15076, Found: m/z 384.1505. IR (in cm-1) 559.15, 578.64, 632.66, 690.79, 722.93, 748.91, 951.67, 1044.33, 1055.27, 1091.86, 1162.98, 1262.13, 1367.79, 1313.76, 1445.40, 1494.98, 1583.54, 1661.15, 2928.99, 2854.22, 3440.50.
0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1100 C, 1H NMR (600 MHz,CDCl3): δ (in ppm) 1.21-1.26 (1H, m), 1.32-1.38 (2H, m), 1.42-1.45 (1H, m), 1.61-1.62 (1H, m), 1.65-1.66 (3H, m), 1.70 (3H, s), 1.86-1.87 (1H, m), 4.46 (1H, d, J = 18), 4.75 (1H, d, J = 18), 6.28 (1H, d, J = 6), 6.82 (1H, d, J = 12), 7.08-7.13 (3H, m), 7.33-7.36 (2H, m), 7.41-7.44 (1H, m), 7.72 (2H, d, J = 6); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.66, 25.09, 25. 19, 27.82, 28.82, 41.21, 43.61, 66.48, 124.44, 124.92, 125.54, 126.41, 127.22, 127.69, 129.00, 132.59, 138.97, 169.06; HRMS (EI+): m/z Calcd for C21H24N2O3S(M)+ 384.15076, Found: m/z 384.1505. IR (in cm-1) 559.15, 578.64, 632.66, 690.79, 722.93, 748.91, 951.67, 1044.33, 1055.27, 1091.86, 1162.98, 1262.13, 1367.79, 1313.76, 1445.40, 1494.98, 1583.54, 1661.15, 2928.99, 2854.22, 3440.50.
1-(3-benzenesulfonyl-2-cyclohexyl-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9f): White solid, Rf  0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1200 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.98-1.10 (4H, m), 1.33-1.35 (2H, m), 1.47-1.50 (2H, m), 1.57-1.61 (2H, m), 1.70 (3H, s), 1.85-1.87 (1H, m), 4.43 (1H, d, J = 18), 4.73 (1H, d, J = 18), 6.24 (1H, d, J = 6), 6.82 (1H, d, J = 6), 7.08-7.12 (3H, m), 7.33-7.36 (2H, m), 7.42 (1H, t, J = 6), 7.72 (2H, d, J = 12); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.43, 25.44, 25.88, 27.95, 28.85, 29.66, 37.85, 43.42, 66.92, 125.58, 126.47, 127.21, 127.62, 129.01, 132.58, 134.79, 139.05, 169.34; HRMS (EI+): m/z Calcd for C22H26N2O3S (M)+ 398.1664, Found: m/z 398.16632. IR (in cm-1) 567.70, 578.64, 621.72, 647.70, 690.79, 718.82, 744.81, 930.13, 964.66, 1042.28, 1091.86, 1152.04, 1169.47, 1175.97, 1260.08, 1324.70, 1352.74, 1378.39, 1447.45, 1456, 1499.43, 1585.59, 1658.76, 2854.22, 2928.99, 3435.
0.50 (petroleum ether, ethyl acetate 3:2), Melting point 1200 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 0.98-1.10 (4H, m), 1.33-1.35 (2H, m), 1.47-1.50 (2H, m), 1.57-1.61 (2H, m), 1.70 (3H, s), 1.85-1.87 (1H, m), 4.43 (1H, d, J = 18), 4.73 (1H, d, J = 18), 6.24 (1H, d, J = 6), 6.82 (1H, d, J = 6), 7.08-7.12 (3H, m), 7.33-7.36 (2H, m), 7.42 (1H, t, J = 6), 7.72 (2H, d, J = 12); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.43, 25.44, 25.88, 27.95, 28.85, 29.66, 37.85, 43.42, 66.92, 125.58, 126.47, 127.21, 127.62, 129.01, 132.58, 134.79, 139.05, 169.34; HRMS (EI+): m/z Calcd for C22H26N2O3S (M)+ 398.1664, Found: m/z 398.16632. IR (in cm-1) 567.70, 578.64, 621.72, 647.70, 690.79, 718.82, 744.81, 930.13, 964.66, 1042.28, 1091.86, 1152.04, 1169.47, 1175.97, 1260.08, 1324.70, 1352.74, 1378.39, 1447.45, 1456, 1499.43, 1585.59, 1658.76, 2854.22, 2928.99, 3435.
1-(2-Cyclopentyl-3-(4-fluoro-benzenesulfonyl)-3,4-dihydro-2H-quinazolin-1-yl)-ethanone (9j): White solid, Rf  0.40 (petroleum ether, ethyl acetate 3:2), Melting point 1500 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 1.32-1.38 (2H, m), 1.42-1.44 (1H, m), 1.51 (3H, s), 1.62-1.67 (3H, m), 1.77 (2H, b s), 1.85-1.87 (1H, m), 4.48 (1H, d, J = 18), 4.68 (1H, d, J = 18), 6.28 (1H, d, J = 12), 6.85 (1H, d, J = 6), 7.01-7.04 (2H, m), 7.08-7.16 (3H, m), 7.76-7.79 (2H, m); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.49, 25.06, 25.18, 27.81, 28.84, 41.29, 43.61, 66.44, 116.06, 116.21, 124.53, 124.91, 125.71, 126.48, 127.38, 130.50, 130.56, 135.03, 164.24, 165.94, 168.98; HRMS (EI+): m/z Calcd for C21H24N2O3S (M)+ 402.1413, Found: m/z 402.1415. IR (in cm-1) 568.96, 621.035, 680.82, 742.54, 808.11, 887.19, 925.76, 977.84, 1033.77, 1099.34, 1132.13, 1157.20, 1242.07, 1311.5, 1394.43, 1450.36, 1587.3, 1654.81, 2354.92, 2856.37, 2918.09, 2950.88, 3550.70, 3770.57.
0.40 (petroleum ether, ethyl acetate 3:2), Melting point 1500 C, 1H NMR (600 MHz, CDCl3): δ (in ppm) 1.32-1.38 (2H, m), 1.42-1.44 (1H, m), 1.51 (3H, s), 1.62-1.67 (3H, m), 1.77 (2H, b s), 1.85-1.87 (1H, m), 4.48 (1H, d, J = 18), 4.68 (1H, d, J = 18), 6.28 (1H, d, J = 12), 6.85 (1H, d, J = 6), 7.01-7.04 (2H, m), 7.08-7.16 (3H, m), 7.76-7.79 (2H, m); 13C NMR (150 MHz, CDCl3): δ (in ppm) 22.49, 25.06, 25.18, 27.81, 28.84, 41.29, 43.61, 66.44, 116.06, 116.21, 124.53, 124.91, 125.71, 126.48, 127.38, 130.50, 130.56, 135.03, 164.24, 165.94, 168.98; HRMS (EI+): m/z Calcd for C21H24N2O3S (M)+ 402.1413, Found: m/z 402.1415. IR (in cm-1) 568.96, 621.035, 680.82, 742.54, 808.11, 887.19, 925.76, 977.84, 1033.77, 1099.34, 1132.13, 1157.20, 1242.07, 1311.5, 1394.43, 1450.36, 1587.3, 1654.81, 2354.92, 2856.37, 2918.09, 2950.88, 3550.70, 3770.57.
Acknowledgment
Moumita Chatterjee wishes to thank UGC, Suvankar Bera wishes to thank CSIR, Mohd Usman Mohd Siddique wishes to thank NIPER-KOLKATA for their respective fellowships. We would also like to thank the CSIR ‘ORIGIN’ CSC-0108 project for providing financial assistance towards this work. The authors would also like to thank the central instrumentation facilities of CSIR-IICB for recording the spectra.
References
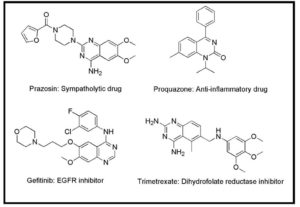
1. ALAGARSAMY, V., RAJA SOLOMON, V. & DHANABAL, K. 2007a. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorganic & Medicinal Chemistry, 15 (1) 235-241.
Publisher – Google Scholar
2. ALAGARSAMY, V., SOLOMON, V. R. & MURUGAN, M. 2007b. Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new class of H1-antihistaminic agents. Bioorganic & Medicinal Chemistry, 15 (12) 4009-4015.
Publisher – Google Scholar
3. BRANDS, M., GRANDE, Y. C., ENDERMANN, R., GAHLMANN, R., KRÜGER, J. & RADDATZ, S. 2003. Pyrimidinone antibiotics–heterocyclic analogues with improved antibacterial spectrum. Bioorganic & Medicinal Chemistry Letters, 13 (16) 2641-2645.
Publisher – Google Scholar
4. CHEN, C.-J., SONG, B.-A., YANG, S., XU, G.-F., BHADURY, P. S., JIN, L.-H., HU, D.-Y., LI, Q.-Z., LIU, F., XUE, W., LU, P. & CHEN, Z. 2007. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorganic & Medicinal Chemistry, 15 (12) 3981-3989.
Publisher – Google Scholar
5. COMBY, F., LAGORCE, J. F., MOULARD, T., BUXERAUD, J. & RABY, C. 1993. [Antibacterial sulfonamides, antiparasitic and antifungal derivatives of imidazole: evaluation of their antithyroid effects in the rat]. Vet Res, 24 (4) 316-26.
6. ELIOPOULOS, G. M. & HUOVINEN, P. 2001. Resistance to Trimethoprim-Sulfamethoxazole. Clinical Infectious Diseases, 32 (11) 1608-1614.
Publisher – Google Scholar
7. GHORAB, M. M., RAGAB, F. A., HEIBA, H. I., YOUSSEF, H. A. & GALA, M. 2010. Synthesis of some new pyrazole and pyrimidine derivatives carrying a sulfonamide moiety of expected antitumor activity and study of the synergistic effect of γirradiation. Arzneimittelforschung, 60(1) 48-55.
8. GIUSEPPE, C., PIERO, D. C., MADDALENA, G. & CONCETTA, L. R. 2010. Tetrahydroquinazoline Derivatives by Aza Diels-Alder Reaction. HETEROCYCLES, 80 (2) 1457-1461.
Publisher – Google Scholar
9. HUOVINEN, P., SUNDSTROM, L., SWEDBERG, G. & SKOLD, O. 1995. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother, 39 (2) 279-89.
Publisher – Google Scholar
10. ISMAIL, M. A. H., BARKER, S., ABOU EL ELLA, D. A., ABOUZID, K. A. M., TOUBAR, R. A. & TODD, M. H. 2006.Design and Synthesis of New Tetrazolyl- and Carboxy-biphenylylmethyl-quinazolin-4-one Derivatives as Angiotensin II AT1 Receptor Antagonists. Journal of Medicinal Chemistry, 49 (5) 1526-1535.
Publisher – Google Scholar
11. KAJINUMA, H.; KUZUYA, T. & IDE, T. 1974. Effects of Hypoglycemic Sulfonamides on Glucagon and Insulin Secretion in Ducks and Dogs. Diabetes, 23 (5) 412-417.
12. KOMBU, R. S., MAILAVARAM, R. P., DEVALAPALLY, H., CHINNAPPA, P. M., DEVARAKONDA, R. K. & AKKINEPALLY, R. R. 2008. Synthesis and Bronchodilator Studies of Some Novel 6-Alkyl/Aryl-1,2,4-Triazino[4,3-c]Quinazolines. Open Med Chem J, 2 101-11.
Publisher – Google Scholar
13. KOREEDA, M. & YANG, W. 1994. Chemistry of 1,2-Dithiins. Synthesis of the Potent Antibiotic Thiarubrine A. Journal of the American Chemical Society, 116 (23) 10793-10794.
Publisher – Google Scholar
14. KUMAR, K. S., GANGULY, S., VEERASAMY, R. & DE CLERCQ, E. 2010. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4(3)H-ones. European Journal of Medicinal Chemistry, 45 (11) 5474-5479.
Publisher – Google Scholar
15. KUNES, J., BAZANT, J., POUR, M., WAISSER, K., SLOSAREK, M. & JANOTA, J. 2000. Quinazoline derivatives with antitubercular activity. Farmaco, 55 (11-12) 725-9.
Publisher – Google Scholar
16. LOMBARDO, L. J., LEE, F. Y., CHEN, P., NORRIS, D., BARRISH, J. C., BEHNIA, K., CASTANEDA, S., CORNELIUS, L. A. M., DAS, J., DOWEYKO, A. M., FAIRCHILD, C., HUNT, J. T., INIGO, I., JOHNSTON, K., KAMATH, A., KAN, D., KLEI, H., MARATHE, P., PANG, S., PETERSON, R., PITT, S., SCHIEVEN, G. L., SCHMIDT, R. J., TOKARSKI, J., WEN, M.-L., WITYAK, J. & BORZILLERI, R. M. 2004. Discovery of N-(2-Chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a Dual Src/Abl Kinase Inhibitor with Potent Antitumor Activity in Preclinical Assays. Journal of Medicinal Chemistry, 47 (27) 6658-6661.
Publisher – Google Scholar
17. MARINI, A. M., MARESCA, A., AGGARWAL, M., ORLANDINI, E., NENCETTI, S., DA SETTIMO, F., SALERNO, S., SIMORINI, F., LA MOTTA, C., TALIANI, S., NUTI, E., SCOZZAFAVA, A., MCKENNA, R., ROSSELLO, A. & SUPURAN, C. T. 2012. Tricyclic Sulfonamides Incorporating Benzothiopyrano[4,3-c]pyrazole and Pyridothiopyrano[4,3-c]pyrazole Effectively Inhibit α- and β-Carbonic Anhydrase: X-ray Crystallography and Solution Investigations on 15 Isoforms.Journal of Medicinal Chemistry, 55 (22) 9619-9629.
Publisher – Google Scholar
18. ÖZBEK, N., KATıRCıOÄžLU, H., KARACAN, N. & BAYKAL, T. 2007. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorganic & Medicinal Chemistry, 15 (15) 5105-5109.
Publisher – Google Scholar
19. ROHINI, R., SHANKER, K., REDDY, P. M., HO, Y.-P. & RAVINDER, V. 2009. Mono and bis-6-arylbenzimidazo[1,2-c]quinazolines: A new class of antimicrobial agents. European Journal of Medicinal Chemistry, 44 (8) 3330-3339.
Publisher – Google Scholar
20. TEMPERINI, C., CECCHI, A., SCOZZAFAVA, A. & SUPURAN, C. T. 2008. Carbonic anhydrase inhibitors. Sulfonamide diuretics revisited-old leads for new applications? Organic & Biomolecular Chemistry, 6 (14) 2499-2506.
Publisher – Google Scholar
21. VOLZHINA, O. N. & YAKHONTOV, L. N. 1982. Quinazoline cardiovascular agents (review). Pharmaceutical Chemistry Journal, 16 (10) 734-741.
22. XIE, M., UJJINAMATADA, R. K., SADOWSKA, M., LAPIDUS, R. G., EDELMAN, M. J. & HOSMANE, R. S. 2010. A novel, broad-spectrum anticancer compound containing the imidazo[4,5-e][1,3]diazepine ring system. Bioorganic & Medicinal Chemistry Letters, 20 (15) 4386-4389.
Publisher – Google Scholar