Introduction
Percutaneous radiofrequency procedures have become a frequently performed treatment option in the management of chronic pain. It is well established in the treatment of back pain of facet joint origin and was increasingly introduced in the treatment of various other chronic pain conditions, amongst which the chronic regional pain syndrome (CRPS) (Manjunath et al., 2008). In patients with CRPS radiof-requency is applied to the lumbar sympa-thetic chain in order to release the so-called sympathetically maintained pain (SMP) (Bergamin et al., 2011).
CRPS is a generic term for a variety of painful conditions and abnormal findings in the upper or lower limbs, which usually occur after a limb trauma or surgery. Two types are distinguished; Type 1 without, and Type 2 with a definable nerve lesion. The affected area and the severity of pain typically exceed the original extent and expected clinical course of the inciting injury. The symptoms consist of continuous pain, hyperalgesia and allodynia, changes in the temperature and the colour of the skin, oedema, abnormal sudomotor activity, trophic changes of the hair and the nails and different signs of motor dysfunction. The symptoms appear in various combinations and may change over time (Harden et al., 2007; van Eijs et al., 2011). The complex pathophysiology, including multiple peripheral and central pathomechanisms that maintain each other in a sort of a vicious circle, is still not completely understood (Groeneweg et al., 2009). Amongst many other mechanisms the sympathetic nervous system is believed to play an important role in pain generation (van Eijs et al., 2011). The sympathetically maintained pain (SMP) is the component of pain that can be relieved by sympathetic blocks. SMP is associated with various pain disorders and appears in about 50% of the patients with CRPS (Groeneweg et al., 2009).
In CRPS-patients with SMP sympathetic blocks of the sympathetic ganglia with local anaesthetics are frequently used. To achieve a longer lasting pain relief the application of CRF instead of a local anaesthetic has been proposed. It seems to be an effective treatment in cases where conservative management has failed and the response to a diagnostic sympathetic block was positive (Manjunath et al., 2008; Groeneweg et al., 2009). The analgesic effect facilitates the restoration of the function of the limb by physiotherapy (Racz and Stanton-Hicks, 2002). However, to reduce the risk of deafferention pain as a side effect of neurolytic procedures, the non-destructive PRF was increasingly introduced in the treatment of SMP (Racz and Stanton-Hicks, 2002; Ahadian, 2004). Despite its frequent use in clinical practice its application is based on very little evidence. There are only three case reports describing PRF for this indication, yet, all achieving a considerable pain relief (Ahadian, 2004; Straube et al., 2010; Kabbara et al., 2003).
There are two basic types of radiofrequency. The thermal or continuous radiofrequency (CRF) produces a well-circumscribed heat lesion in the target tissue. It is confirmed as a safe and atraumatic procedure with a low complication rate (Kornick et al., 2004). However, the fact that the high temperature around the electrode coagulates the nerve, contraindicates the use of CRF to treat neuropathic pain syndromes (Cohen et al., 2010).
With the development of pulsed radiofre-quency (PRF) (Sluijter et al., 1998) a non-destructive radiofrequency method and therefore suitable to treat neuropathic pain became available (Munglani et al., 1999). In contrast to CRF the RF-current is delivered in short pulses. The silent phase between the bursts allows the heat to dissipate in order to keep the heat at the electrode tip below the neurodestructive temperature of 43°C. CRF requires an exact and parallel electrode placement, and usually several heat lesions at slightly different positions to secure that the nerve is completely interrupted and to consider variations in its anatomical course. It is therefore a more time-consuming and laborious procedure than PRF. Since PRF proved to be safe at is easier to perform, it was very quickly introduced in clinical practice (Bogduk 2006). Nevertheless, the research about the biological effects is considerably lagging behind (Cohen et al., 2010; Bogduk, 2006) and the mechanisms responsible for the analgesic effect of PRF are still not completely understood.
The low electromagnetic fields induced by the RF-current seem to play an important role in neuromodulation and alterations in synaptic transmission, which may account for the pain relief. On the one hand, they might induce a long-term depression of the synaptic transmission in the spinal cord that antagonizes the long-term potentiation influencing the processing of sensory in-formation in chronic pain states. On the other hand, they seem to have an impact on different transcription factors in the neurons (Chuha et al., 2011) Those findings are reflected in several animal trials where PRF could significantly relieve artificially in-duced neuropathic pain when applied to the dorsal root ganglion or peripheral nerves (Aksu et al., 2010; Hagiwara et al., 2009). Interestingly, in one of the studies the anal-gesic effect could be attenuated by the intrathecal application of antiadrenergic drugs. Descending noradrenergic and serotonergic inhibitory pathways are known to be involved in mediating endogenous analgesia. Thus, PRF might exert a part of its analgesic effect through an enhancement of those pathways (Hagiwara et al., 2009). If and in what ways these findings might play a role in the clinical effect of PRF still needs to be established.
For the frequently performed lumbar sym-pathetic blocks (LSB) with pulsed radiofre-quency in CRPS patients in our clinic, there is almost no literature available. It was therefore a major topic to analyse prelimi-nary data to evaluate the effectiveness for pulsed radiofrequency in the treatment of this disease.
Materials and Methods
Patients and Materials
Between 2004 and 2010 a prospective evaluation of radiofrequency treatment for CRPS was completed at the University Hos-pital in Zürich. Consecutive patients with CRPS of the lower extremities that did not respond to conservative management and who fulfilled the inclusion criteria under-went radiofrequency treatment.
The inclusion criteria consisted in a positive response to a local anaesthetic block of the lumbar sympathetic chain in patients with a clinically diagnose CRPS. Age older than 18 years was required. The exclusion criteria comprised allergies to contrast media or local anaesthetics, coagulation disturbances, infections, mental handicap or psychiatric disorder impairing adequate communication or cooperation, severe anatomical aberrations that could affect the safety or success of the procedure, and pregnancy.
Written informed consent was obtained from all patients and the study was approved by the ethical review board responsible for our institution (Kantonale Ethikkommission Zürich).
Methods
Prior to PRF-treatment of the lumbar sym-pathetic ganglia in patients with CRPS, one ore more diagnostic/therapeutic lumbar sympathetic blocks (LSB) with local anaes-thetics were performed at different occa-sions. A significant pain relief or change in surface temperature, measured by a surface thermometer, of the affected extremity after the block, is an indication that sympa-thetically maintained pain is present (Har-trick et al., 2004), which was the case in 15 patients.
The blocks were performed under aseptic conditions, fluoroscopic guidance and local skin anaesthesia without sedation. The patient was lying prone on a table. For the diagnostic blocks the C-arm intensifier was turned in an oblique position until the distal end of the processus transversus was projected on the lateral edge of L3 vertebral body. The lumbar sympathetic ganglia are located at the anterolateral side of the vertebral body. The cannula was inserted until the tip reached the anterior border of the corpus vertebrae. Correct position and possible venous uptake were verified with fluoroscopic antero-posterior and lateral views. The lateral view served to ensure that the contrast medium did spread as a thin line along the anterior aspect of the psoas fascia and the vertebral body and that the needle tip did not pass the anterior border of the latter (van Eijs et al., 2011). If there was a clear outline of the contrast spread, no further needle approach at L2 or L4 was made. For the diagnostic LSB 10ml of 0.375% bupi-vacaine and 3ml of lidocaine 1% was injected through a 20 gauge, 150mm needle.
PRF treatment was performed with a 20 gauge RF-cannula with a 10mm active tip. A small amount of lidocaine 1% for local skin anaesthesia was injected. PRF-current with a pulse duration of 20ms at a frequency of 2Hz was applied for 2 min twice on three different levels and a electrode tip tempera-ture not exceeding 42°C (Bogduk, 2006).
Outcome Measurements
The patients filled in a simple questionnaire that was designed for this particular investigation, before treatment, six weeks after and six months after the intervention. It was sent to the patients after the consul-tation and they filled it in at home. A neutral person who didn’t have any contact with the patients did the evaluation. The questionnaire contained 11-point numeric rating scales (NRS, 0=not at all/ never, 10= worst/always) to record different pain parameters (present pain, highest and lowest pain intensity during the last week, average pain during the last week), improvement in the quality of life and the satisfaction with the procedure (0=not at all satisfied, 10=very satisfied). The impairment of different daily activities (to get dressed, uplifting something, running and walking, sitting, standing, sleeping, travelling, social life and leisure time) were documented with a 6-point NRS. Prior to the procedure the patients desired pain relief could be noted on a scale in percentages. All patients noted their analgesic intake before and after treatment, which was recorded by the 3 steps of the WHO-ladder (0=no analgesics, 1= non-opioids, 2= non-opiods + weak opioids, 3= non-opioids + strong opioids). The general emotional state was also de-tected by a 4-point scale (0= feeling not at all depressed, 4 = feeling very depressed) during the follow-up.
Primary outcome of the trial were the me-dian changes in the different pain and daily activity scores, as well as the improvement in the quality of life.
Statistics
Data were analyzed using the statistics program SPSS (PASW Statistics 18.0, SPSS Inc. Hong Kong, China). Descriptive statistics of all variables were computed. The significance of differences between follow-up scores to baseline values was evaluated by the non-parametric Wilcoxon-test. A two-tailed p value less than 0.05 was considered statistically significant.
Results
Demographics and Complications
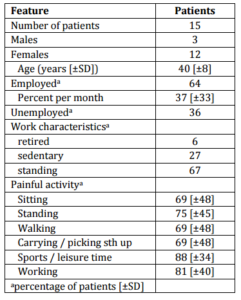
All 15 patients completed the 6 week-evaluation, but only 8 the 6 months follow-up. The mean age was 40 (±8) years. No complications occurred. Demographic and clinical data are given in Table 1.
Table 1. Demographic and Clinical Data of the Treated Patients
Outcome
The outcomes are summarized in Table 2. At 6 weeks the scores of the present, heaviest and average pain were significantly reduced, apart from the lowest pain-intensity. The relief was even more pronounced at 6 months, but did not stay statistically significant. The quality and quantity of pain in the leg did not show a significant reduc-tion. A distinct improvement in the quality of life could be seen during the whole follow-up, but it just failed the significance level. The daily activities running/walking and uplifting something showed a significant improvement at 6 weeks. A trend could be seen in the improvement of sleeping and social life/leisure time. All the other activities did not change significantly. The analgesic intake was not significantly reduced either. The level of feeling de-pressed was low at 6 weeks (0.5 (0.9), p= 0.047) and 6 months (0.4 (0.7), p= 0.197). The average pain relief was 15% at 6 weeks and 13% at 6 months compared to a desired relief of almost 70%. However, the satisfaction with the procedure was relatively high (7.5 (2.2) p<0.001, 7.8 (3.2) p<0.001). (Figure 1)
Table 2. Data of Patients with Complex Regional Pain Syndrome (CRPS). Mean Preproce-dural and Postprocedural Values. For the Outcome Measures Evaluated before and after the RF-Procedure, p Values are Given for the Differences between the Baseline-Value and the Follow-up.
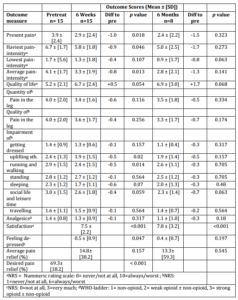
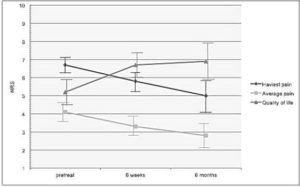
Figure 1. Changes of the Average and the Heaviest Pain-Intensity Compared to the im-provement in the Quality of Life in Patients with Complex Regional Pain Syndrome (CRPS). The Vertical Lines Represent the SEM. NRS= Numeric Rating Scale; 1= No Pain; 10= Worst Pain.
Discussion
The main purpose of this investigation was to evaluate the effectiveness of the RF-procedures within a routine clinical setting in the University Hospital of Zürich. A major shortcoming of this study consists in the small number of patients some missing data about the follow-up. Unfortunately the follow-up was not pursued until one year after the treatment, due to an insufficient number of data available for this period.
For PRF treatment of sympathetically maintained pain in CRPS there is almost no literature available despite its frequent use in clinical practice. There are only three case reports describing PRF for this indication (Ahadian, 2004; Straube et al., 2010; Kabbara et al., 2003). In a patient with CRPS after spinal surgery pain and hyperalgesia decreased from 95 to 25 (- 73.6%) in VAS. The pathologic changes disappeared after 3 days and the clinical effect lasted during the 4 months follow-up (Straube et al., 2010). 50% release lasting for 3 months was achieved in two patients in a further case report (Kabbara et al., 2003). In a retrospective analysis with 12 patients, 7 (58%) experienced good to excellent results at the 3 months follow-up. Unfortunately, the terms “excellent” and “good” were not defined more precisely (Ahadian, 2004).
The present evaluation showed a significant treatment effect in most of the pain scores and some of the daily activities at 6 weeks, but did not stay significant until 6 months. Although the effect size was rather small and the average pain relief only amounted to 15% and 13% respectively, the patients were highly satisfied with the procedure during the whole follow-up and the improvement of quality of life missed the significance level by little. Correspondingly the need for analgesics was similar before and after the treatment. However, the WHO ladder may not a tool sensitive enough to detect smaller alterations in the need for analgesics, because it only records a change of the substance classes but not a change of the amount of their daily requirement.
Up to now the pain relief with PRF applica-tion seems to be slightly less in size and duration compared to what can be achieved with CRF (Manjunath et al., 2008). What makes PRF undoubtedly more favourable than CRF is its non-destructive nature (Munglani, 1999; Bogduk, 2006). The risk of deafferentation syndrome or injuries to adjacent nerve structures as possible complications of CRF can be reduced (Racz and Stanton-Hicks, 2002; Ahadian, 2004). Indeed, up to date, in none of the evaluations of PRF treatment of the lumbar sympathetic chain – including the actual evaluation – such side effects have been reported (Ahadian, 2004; Straube et al., 2010; Kabbara et al., 2003).
Conclusion
In the difficult and challenging treatment of CRPS, pulsed radiofrequency seems to be a promising and safe treatment option. It is able to offer a significant pain relief and to improve the disability for at least 6 weeks in patients that didn’t respond to conservative treatment. This evaluation is based on a real patient population selected after criteria that are common in clinical practice. However, neither the use in clinical practice nor the mode of action of PRF are validated yet and further studies are urgently needed to clarify its role in the interventional pain management and to establish uniform procedure guidelines.
Acknowledgments
The authors thank Mrs. Diana Haag for the grammatical correction of the manuscript.
References
Ahadian, F. M. (2004). “Pulsed Radiofre-Quency Neurotomy: Advances in Pain Medi-Cine,” Current Pain and Headache Reports, 8 (1) 34-40.
Publisher – Google Scholar
Aksu, R., Uğur, F., Bicer, C., Menkü, A., Güler, G., Madenoğlu, H. et al. (2010). “The Efficiency of Pulsed Radiofrequency Application on L5 and l6 Dorsal Roots in Rabbits Developing Neuropathic Pain,” Regional Anesthesia & Pain Medicine, 35 (1) 11–5.
Publisher – Google Scholar
Bergamin, N., Aeschbach, A., Michel, B. A. & Sprott, H. (2011). “Radiofrequency Therapy in Back Pain and Complex Regional Pain Syndrome (CRPS),” Rheumatology Reports. [Online], [Retrieved November 9, 2011],
Publisher – Google Scholar
Bogduk, N. (2006). “Pulsed Radiofrequency,” Pain Medicine, 7 (5) 396-407.
Publisher – Google Scholar
Chua, N. H. L., Vissers, K. C. & Sluijter, M. E. (2011). “Pulsed Radiofrequency Treatment in Interventional Pain Management: Mech-Anisms and Potential Indications – A Review,” Acta Neurochirurgica (Wien), 153 (3) 763-71.
Publisher – Google Scholar
Cohen, S. P. & Van Zundert, J. (2010). “Pulsed Radiofrequency: Rebel without Cause,” Reg Anesth Pain Med, 35 (1) 8-10.
Publisher – Google Scholar
Groeneweg, G., Huygen, F. J. P. M., Coderre, T. J. & Zijlstra, F. J. (2009). “Regulation of Peripheral Blood Flow in Complex Regional Pain Syndrome: Clinical Implication for Symptomatic Relief and Pain Management,” BMC Musculoskeletal Disord, 10 (23) 116.
Publisher – Google Scholar
Hagiwara, S., Iwasaka, H., Takeshima, N. & Noguchi, T. (2009). “Mechanisms of Analge-Sic Action of Pulsed Radiofrequency on Ad-Juvant-Induced Pain in the Rat: Roles of Descending Adrenergic and Serotonergic Systems,”European Journal of Pain, 13 (3) 249-52.
Publisher – Google Scholar
Harden, R. N., Bruehl, S., Stanton-Hicks, M. & Wilson, P. R. (2007). “Proposed New Diagnostic Criteria for Complex Regional Pain Syndrome,” Pain Medicine, 8 (4) 326-31.
Publisher – Google Scholar
Hartrick, C. T., Kovan, J. P. & Naismith, P. (2004). “Outcome Prediction Following Sympathetic Block for Complex Regional Pain Syndrome,” Pain Practice, 4 (3) 222-8.
Publisher – Google Scholar
Kabbara, A. I., Chelimsky, T. & Boswell, M. V. (2003). ‘Paresthesiae during Radiofre-Quency Neurolysis of Lumbar Sympathetic Trunk in CRPS: Human Evidence of a Sympa-Tho-Sensory Connection?,”’Pain Physician, 6 (4) 421-4.
Kornick, C., Kramarich, S. S., Lamer, T. J. & Todd Sitzman, B. (2004). “Complications of Lumbar Facet Radiofrequency Denervation,” Spine, 29 (12) 1352-4.
Publisher – Google Scholar
Manjunath, P. S., Jayalakshmi, T. S., Dureja, G. P. & Prevost, A. T. (2008). “Management of Lower Limb Complex Regional Pain Syndrome Type 1: An Evaluation of Percutaneous Radiofrequency Thermal Lumbar Sympa-Thectomy Versus Phenol Lumbar Sympathetic Neurolysis – A Pilot Study,” Anesthesia & Analgesia, 106 (2) 647-9.
Publisher – Google Scholar
Munglani, R. (1999). “The Longer Term Effect of Pulsed Radiofrequency for Neuropathic Pain,” Pain, 80 (1-2) 437-9.
Publisher – Google Scholar
Racz, G. B. & Stanton-Hicks, M. (2002). “Lumbar and Thoracic Sympathetic Radiof-Requency Lesioning in Complex Regional Pain Syndrome,” Pain Practice, 2 (3) 250-6.
Publisher – Google Scholar
Sluijter, M., Cosman, E., Rittman, I. & van Kleef, M. (1998). ‘The Effects of Pulsed Ra-Diofrequency Field Applied to the Dorsal Root Ganglion – A Preliminary Report,’ Pain Clin, 11 (2) 109–17.
Straube, S., Derry, S., Moore, R. A. & McQuay, H. J. (2010). “Cervico-Thoracic Or Lumbar Sympathectomy for Neuropathic Pain and Complex Regional Pain Syndrome,” Cochrane Database of Systematic Reviews, 7 (7) CD002918. Review.
Publisher – Google Scholar
van Eijs, F., Stanton-Hicks, M., van Zundert, J., Faber, C. G., Lubenow, T. R., Mekhail, N. et al. (2011). “Evidence-Based Interventional Pain Medicine according to Clinical Diagnosis. 16. Complex Regional Pain Syndrome,” Pain Practice, 11 (1) 70-87.
Publisher – Google Scholar






