Introduction
Ovarian cancer occupies the third rank among cancer in women in Tunisia and its incidence is about 4,1 cases/100000 women [Ben Abdallah, 2012]. The majority of patients diagnosed with ovarian cancer present advanced-stage disease, indeed it is mostly symptomless in early stages and there are currently no effective screening methods. Although, hormonal factors, inflammation, and wound healing are thought to play important roles in its occurrence, the etiology of ovarian cancer remains unknown [Risch, 1998]. Many genetic association studies have been already performed in cancer and suggested that tumor predisposition may be due to the combination of low penetrance genetic variants [Balmain, 2003; Kotnis, 2005] including genes coding for pro-inflammatory cytokines and their receptors [Balkwill, 2001; Martin Howell, 2007]. Interleukin-1 (IL-1), an inflammatory regulator, plays an important role in the development of several pathologies [Alrayes, 2003; Langdahl, 2000]. The IL-1 gene family is located on chromosome 2q and includes IL-1A, IL-1B, and IL-1RN genes, which encode IL-1α, IL-1 β, and IL-1 receptor antagonist (IL-1ra) [Hefler, 2002]. This receptor is in anti-inflammatory cytokine which acts as competitive inhibitor to control the inflammatory action of IL-1 by binding to the IL-1 receptor [Arend, 1998]. Within the IL-1RN gene, a variable number of tandem repeats (VNTR) of 86-bp length was found to correlate with many diseases [Alrayes, 2003; Langdahl, 2000; Al-Moundhri, 2006; Sehouli, 2002]. Nevertheless, there are limited reports on the influence of this variant with host susceptibility to ovarian cancer and the results are controversial [Sehouli, 2003; Hefler, 2002].
In the present study, we investigated the association between the IL-1RN VNTR polymorphism and susceptibility to ovarian cancer and its correlation with established clinical prognostic factors in Tunisian patients in North Africa (figure1).
Figure 1: Geographic Localisation of Tunisia (The Study Population)
Materials and Methods
Sample Collection
All blood samples were collected from histopathological confirmed Tunisian patients with ovarian cancer (n=55) and unrelated, healthy female controls of similar ethnicity (n=257). Clinical diagnosis/staging of ovarian cancer patients was performed by trained medical personnel as per the guidelines outlined by the International Federation of Gynecology and Obstetrics (FIGO). Written informed consent was taken from all subjects and the study was approved by local ethics committees of the Salah Azeiz Institute of oncology.
Blood Collection and DNA Extraction
Five milliliters of venous blood with EDTA, as an anticoagulant, were collected from each subject. The blood was obtained from patients prior to radiation therapy or chemotherapy. Genomic DNA was extracted using QIAamp® DNA blood Mini Kit (QiagenGmbH, Hilden).
IL-1RN Gene Polymorphism
The polymorphic region within the second intron of IL-1RN, which contains a VNTR of 86-pb, was amplified by polymerase chain reaction with specific primers: forward, 5’ CTCCAGCAACACTCCTAT 3’; reverse, 5’ TCCTGGTCTGCAGGTAA 3’. For each sample, the PCR was performed in a total reaction volume of 10ml containing 1ml (100 ng) genomic DNA, 25mM of dNTP, 1µl of buffer 10X and 0,25U of Taq polymerase (Fermentas). The PCR cycling program comprised an initial denaturation at 95 ◦C for 10 minutes, followed by 30 cycles of 95 ◦C for 20 seconds, annealing of primers at 51 ◦C for 30 seconds and extension at 72 ◦C for 50 seconds and a final extension at 72°C for 10 minutes. Amplified DNA fragments were separated on 1.5% ethidium bromide agarose gel, and visualized under ultraviolet light.
Statistical Analysis
Allele and genotype frequencies were calculated by direct counting and compared with the c2 or Fisher’s exact test when appropriate (e.g., the number of subjects in a cell was less than 5) using the EPI INFO 6 package program (http://wwwn.cdc.gov/epiinfo/html/downloads.htm) and SPSS 17.0 statistic software system (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.).
The odds ratios (OR) and 95% confidence intervals (95% CI) were also calculated in order to measure the strength of the association of individual alleles or genotypes with risk of ovarian cancer, in general, or their FIGO stage and histological type, in particular. A value of P<0.05 was considered statistically significant.
Results
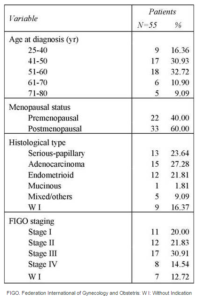
The characteristics of women with ovarian cancer are summarized in Table 1. The median age of patients was 51.80 years (range 25-78). Of 55 patients, 40% have a premenopausal statute and 60% are postmenopausal. The most common histological type was Adenocarcinoma (27.28%). Only 20% were diagnosed in FIGO stage I, 21.83% were in FIGO stage II and 45.45% were in FIGO stage III-IV.
Table 1: Clinicopathological Characteristics of Tunisian Ovarian Cancer Woman Patients
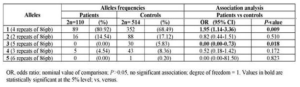
Allelic frequencies of IL-1RN for patients with ovarian cancer and healthy women are given in Table 2. Five different alleles of 86-base pair repeat were detected (allele 1= 4 repeats, allele 2= 2 repeats, allele 3= 5 repeats, allele 4= 3 repeats and allele 5= 6 repeats). Allele 1 was the most common allele in cases and healthy controls with respectively 80.92 and 68.49 % frequencies. The comparison of the allelic distribution of IL-1RN VNTR polymorphism has revealed a significant positive association between allele 1 and the occurrence of ovarian cancer (p=0.009; OR: 1.95, 95% CI, 1.14-3.36) and a negative association between allele 3 (p=0.018. OR: 1.95, 95% CI, 0.00-0.73) and this pathology (table 2).
Table 2: Interleukin-IRN VNTR Alleles in Ovarian Cancer Patients and Controls: Frequencies and Association Analysis
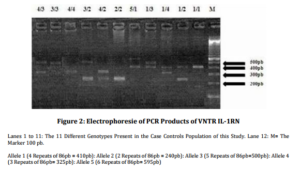
The different combination between the five identified alleles gives 15 genotypes for VNTR IL-1RN polymorphism. But in the present study only 11 genotypes were observed (figure 2).
The distribution of pooled genotypes between carriers and non carriers of allele 1 shows a p value<0,005 however the confidence interval at 95% for odds ratio includes 1, so this association is not statistically significant (table 3).
Tabl 3: Interleukin-IRN VNTR Genotypes in Ovarian Cancer Patients and Controls: Frequencies and Association Analysis
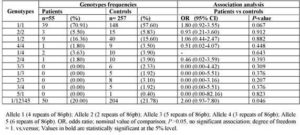
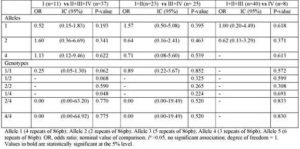
Furthermore, when stratified the cases by histological type, we detect a positive association between allele 1 (p= 0.002; OR: 11.51, 95% CI, 1.65-230.19) and genotype 1/1 (p= 0.012; OR: 8.84, 95% CI, 1.17-184.66) and the susceptibility to Serious-papillary type of ovarian cancer (table 4). In contrast, IL-1RN VNTR alleles and genotypes did not correlate with tumour stage (table 5).
Table 4: Interleukin-IRN VNTR Genotypes in Patients Stratified by Histological Type of Ovarian Cancer and Controls: Frequencies and Association Analysis
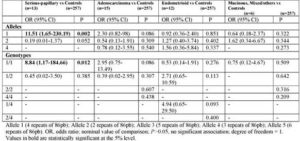
Table 5: Interleukin-IRN VNTR Alleles and Genotypes in Overian Cencer Patients Stratified by Tumour Stage: Frequencies and Assciation Analysis
Discussion
Many studies have indicated that IL-1 plays a key role in cancer’s development [Dinarello, 1996; Fujiwaki, 1997; Viet, 2005]. The role of this cytokine as a mediator of malignant tumour growth has been reported for cervical [Fujiwaki, 1997; Zeisler, 1998], ovarian [Li, 1992], gastric [El Omar, 2001], and colorectal carcinogenesis [Viet, 2005].
Several studies have investigated the role of a common IL-1 RN VNTR polymorphism in the development of inflammatory disorders [Viet, 2005; Fujiwaki, 2003]. The majority of these studies have been enrolled on cancers, such as gastric [Al-Moundhri, 2006; Geon Shin, 2008], bladder [Bid, 2006], breast [Lee, 2007], colorectal [Viet, 2005], cervical [Sousa, 2012; Fujiwaki, 2003], vulvar [Grimm, 2004], and ovarian [Sehouli, 2003; Hefler, 2002]. Nevertheless, there are controversial results regarding the potential role of this polymorphism in the development of cancer. In the present study, we attempted to establish an association between the IL-1RN VNTR polymorphism and susceptibility to ovarian cancer and its correlation with established clinical prognostic parameters in Tunisian women.
In our study, carriers of allele 1 have an almost twofold increased risk of developing ovarian cancer specifically it’s associated to Serous-papillary type susceptibility. This allele seems to be the critical point in the molecular pathway of some diseases. In fact, it has recently been correlated with a higher risk of Multidrug-resistant Acinetobacter baumannii associated pneumonia [Hsu, 2012]. In literature, the presence of allele 1 is associated in general with a restricted immune reaction and the level of IL-1ra production in human endothelial cells with genotype 1/1 is three times higher than with 2/2 genotypes. However, the presence of allele 2 is correlated with a prolonged immune reaction [Dewberry, 2000]. The other alleles are rarely detected and positive or negative effect in inflammatory reaction is not elsewhere described [Witkin, 2002]. Contrary to our results, however, Hefler et al. [2002] have published a case-control study where IL1-RN VNTR polymorphism was genotyped in 94 ovarian cancer patients and 134 healthy women in the Austrian population and found no differences in the prevalence of the VNTR polymorphism in the IL-1RN between cases and controls. However, in Germany, Sehouli et al. [2003] have analysed this polymorphism in 108 women with ovarian cancer compared to 112 patients with benign gynaecological diseases and obtain results suggesting that the allele 2 seems to play a role in the occurrence of ovarian cancer.
Otherwise, our results have revealed, for the first time, a negative association between allele 3 and the occurrence of ovarian cancer in Tunisian women. This allele is rarely detected and his biological meaning on immune reaction is not elsewhere elucidated [Witkin, 2002].
Therefore, the VNTR polymorphism in the IL-1RN should be analyzed in further case-control studies in order to better evaluate this marker as a risk factor for ovarian cancer. Otherwise it needs to be pointed out that there were some limitations in this study. First, the limited sample size may restrict us to identify other genotype or allele associations. Second, our study lacked the measurement of IL-1ra and IL-1β levels in the local environment of ovarian cancer and the representative of circulating IL-1ra and IL-1β levels need to be investigated in further studies. Furthermore, power analysis showed that to achieve a power of 80% at an alpha of 0,05 another 81729 patients would have to be genotyped, in light of the observed allelic frequencies.
In addition, we didn’t observe any association between specific alleles and clinical features such as FIGO stage and histological types. Similar results were obtained by Hefler et al. [2002] and Sehouli et al. [2003]. It can thus speculate that IL-1 RN VNTR polymorphism doesn’t influence ovarian cancer biology.
In conclusion, we observed a significant association of the allele 1 with Serous-papillary type and in the susceptibility to ovarian cancer in Tunisian women. However, the allele 3 is a protective factor for this pathology in Tunisia.
Acknowledgments
We would like to thank to the staff of Tunisian Slah Azeiz Oncology Institute for their collaboration in the collection of blood samples and all blood donors and women with ovarian cancer who participated in the present study. We also thank the staff of the laboratory of Immunology at Military Hospital of Tunis for their collaboration.
References
Al-Moundhri, M. S., Al-Nabhani, M., Al-Bahrani, B., Burney, I. A., Al-Madhani, A., Ganguly, S. S., Al-Yahyaee, S. A. & Grant, C. S. (2006). “Interleukin-1β Gene (IL-1B) and Interleukin 1 Receptor Antagonist Gene (IL-1RN) Polymorphisms and Gastric Cancer Risk in an Omani Arab Population,” Gastric Cancer, 9, 284-290.
Publisher – Google Scholar
Alrayes Hlimy, M. et al. (2003). “Interleukin –1 Receptor Antagonist Gene Polymorphism and Malignant lymphomas,” The Egyptian Journal of Hospital Medicine, 11, 41-49.
Publisher
Arend, W. P., Malyak, M., Guthridge, C. J. & Gabay, C. (1998). “Interleukin-1 Receptor Antagonist: Role In Biology,”Annual Review of Immunology, 16, 27-55.
Publisher – Google Scholar
Balkwill, F. & Mantovani, A. (2001). “Inflammation and Cancer: Back to Virchow?,” The Lancet, 357, 539-545.
Publisher – Google Scholar
Balmain, A., Gray, J. & Ponder, B. (2003). “The Genetics and Genomics of Cancer,” Nature Genetics, 33, 238–244.
Publisher – Google Scholar
Ben Abdallah, M. Ben Yacoub, H. W. Jouini, S. Hsairi, M. & Achour, N. (2012). Registre des Cancers NORD-TUNISIE Données 2004-2006, Service d’Epidémiologie, Biostatistique et Informatique Médicale, Institut Salah AZAIEZ, Tunis, Tunisie.
Bid, H. K., Manchanda, P. K. & Mittal, R. D. (2006). “Association of Interleukin-1 Ra Gene Polymorphism in Patients with Bladder Cancer: Case Control Study from North India,” Urology, 67, 1099-1104.
Publisher – Google Scholar
Dewberry, R., Holden, H., Crossman, D. & Francis, S. (2000). “Interleukin-1 Receptor Antagonist Expression in Human Endothelial Cells and Atherosclerosis,” Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 2394-2400.
Publisher – Google Scholar
Dinarello, C. A. (1996). “Biologic Basis for Interleukine 1 in Disease,” Blood, 87, 2095-2147.
Publisher – Google Scholar
El Omar, E. M., Chow, W. H., Gammon, M. D. et al. (2001). ‘Pro-Inflammatory Genotypes of IL-1B, Tnfa and IL-10 Increase Risk of Distal Gastric Cancer but Not of Cardia or Esophageal Adenocarcinoma,’ Gastroenterology,120 (A) 86.
Fujiwaki, R., Hata, T., Miyazaki, K., Kawamura, T. & Inada, K. (1997). “Elevation of Serum Interleukin-1 Receptor Antagonist Levels in Women with Gynecological Cancers,” BJOG: An International Journal of Obstetrics & Gynaecology,104, 1407–1408.
Publisher – Google Scholar
Fujiwaki, R., Iida, K., Nakayama, K. et al. (2003). “Clinical Significance of Interleukin-1 Receptor Antagonist in Patients with Cervical Cancer,” Gynecologic Oncology, 89, 77-83.
Publisher – Google Scholar
Geon Shin, W., Jang, J. S., Su Kim, H., Jung Kim, S., Ho Kim, K., Kuk Jang, M., Heon Lee, J., Jung Kim, H. & Yang Kim, H. (2008). “Polymorphisms of Interleukin-1 and Interleukin-2 Genes in Patients with Gastric Cancer in Korea,”Journal of Gastroenterology and Hepatology, 23, 1567-1573.
Publisher – Google Scholar
Grimm, C., Berger, I., Tomovski, C., Zeillinger, R., Concin, N., Leodolter, S., Koelbl, H., Tempfer, C. B. & Hefler, L. A. (2004). “A Polymorphism of the Interleukin-1 Receptor Antagonist Plays a Prominent Role within the Interleukin-1 Gene Cluster in Vulvar Carcinogenesis,” Gynecologic Oncology, 92, 936-940.
Publisher – Google Scholar
Hefler, L. A., Ludwig, E., Lebrecht, A., Zeillinger, R., Tong-Cacsire, D., Koelbl, H., Leodolter, S. & Tempfer, C. B. (2002). “Polymorphisms of the Interleukin-1 Gene Cluster and Ovarian Cancer,” Journal of the Society for Gynecologic Investigation, 9, 386-390.
Publisher – Google Scholar
Hsu, M. J., Lu, Y. H., Hsu, Y. C., Liu, W. S. & Wu, W. T. (2012). “Interleukin-1 Receptor Antagonist Gene Polymorphism in Patients with Multidrug-Resistant Acinetobacter Baumannii-Associated Pneumonia,” Annals of Thoracic Medicine, 7, 74-77.
Publisher – Google Scholar
Kotnis, A., Sarin, R. & Mulherkar, R. (2005). “Genotype, Phenotype and Cancer: Role of Low Penetrance Genes and Environment in Tumour Susceptibility,” Journal of Biosciences, 30, 93-102.
Publisher – Google Scholar
Langdahl, B. L., Lokke, E., Carstens, M., Lotte Stenkjaer, L. & Fink Eriksen, E. (2000). “Osteoporetic Fractures are Associated with an 86-Base Pair Repeat Polymorphism in the Interleukin-1 Receptor Antagonist Gene but Not with Polymorphisms in the Interleukin-1b Gene,” Journal of Bone and Mineral Research, 15, 402-414.
Publisher – Google Scholar
Lee, K. M. et al. (2006). “Genetic Polymorphisms of Interleukin-1 Beta (IL-1B) and IL-1 Receptor Antagonist (IL-1RN) and Breast Cancer Risk in Korean Women,” Breast Cancer Research and Treatment, 96 (2) 197-202.
Publisher – Google Scholar
Li, B.- Y., Mohanraj, D., Olson, M. C. et al. (1992). “Human Ovarian Epithelial Cancer Cells Cultured in Vitro Express Both Interleukin 1 α and β Genes,” Cancer Research, 52, 2248-2252.
Publisher
Lindmark, F., Zheng, S. L., Wiklund, F., Balter, K. A., Sun, J., Chang, B., Hedelin, M., Clark, J., Johansson, J. E. et al. (2005). “Interleukin-1 Receptor Antagonist Haplotype Associated with Prostate Cancer Risk,” British Journal of Cancer,93, 493-497.
Publisher – Google Scholar
Martin Howell, W. & Rose-Zerilli, M. J. (2007). “Cytokine Gene Polymorphisms, Cancer Susceptibility, and Prognosis,”The Journal of Nutrition, 137, 194-199.
Publisher – Google Scholar
Risch, H. A. (1998). “Hormonal Etiology of Epithelial Ovarian Cancer, with a Hypothesis Concerning the Role of Androgens and Progesterone,” Journal of the National Cancer Institute, 90, 1774-1786.
Publisher – Google Scholar
Sehouli, J., Mustea, A., Koensgen, D., Chen, F. & Lichtenegger, W. (2003). “Interleukin-1 Receptor Antagonist Gene Polymorphism is Associated with Increased Risk of Epithelial Ovarian Cancer,” Annals of Oncology, 14, 1501-1504.
Publisher – Google Scholar
Sehouli, J., Mustea, A., Koensgen, D., Katsares, I. & Lichtenegger, W. (2002). “Polymorphism of IL-1 Receptor Antagonist Gene: Role in Cancer,” Anticancer Research, 22: 3421-3424.
Publisher – Google Scholar
Sousa, H., Santos, A. M., Catarino, R., Pinto, D., Moutinho, J., Canedo, P., Machado, J. C. & Medeiros, R. (2012). “IL-1RN VNTR Polymorphism and Genetic Susceptibility to Cervical Cancer in Portugal,” Molecular Biology Reports, 39, 10837-10842.
Publisher – Google Scholar
Viet, H. T. et al. (2005). “Interleukin-1 Receptor Antagonist Gene Polymorphism in Human Colorectal Cancer,” Oncology Reports, 14 (4), 915-918.
Publisher – Google Scholar
Witkin, S. S., Gerber, S. & Ledger, W. J. (2002). “Influence of Interleukin-1 Receptor Antagonist Gene Polymorphism on Disease,” Clinical Infectious Diseases, 34, 204–9.
Publisher – Google Scholar
Xue, J. et al. (2010). “No Association of Interleukin-1 Receptor Antagonist VNTR Polymorphism and Rheumatoid Arthritis Susceptibility: A Meta-Analysis,” Clinical and Experimental Rheumatology, 28 (5), 654-660.
Publisher – Google Scholar
Zeisler, H., Tempfer, C., Joura, E. A. et al. (1998). “Serum Interleukin 1 in Ovarian Cancer Patients,” European Journal of Cancer, 34, 931-933.
Publisher – Google Scholar










