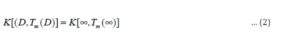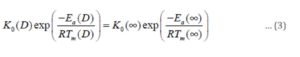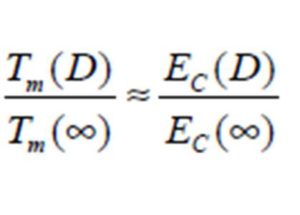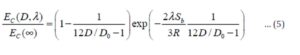Conclusion
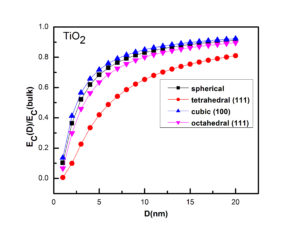
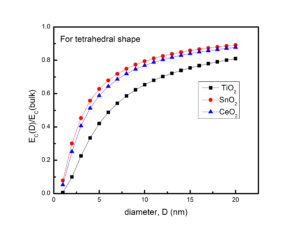
In summary, we have calculated the shape and size dependent catalytic activation energy of TiO2, SnO2 and CeO2 nanostructures. The catalytic activation energy decreases with the decrease in particles’ size. It becomes almost size independent when size of the particle exceeds 10 to 15 nm. We observe that zero dimensional (i.e., spherical) nanoparticles have minimum catalytic activation energy than the two and one dimensional structures. The considerable difference is found for EC in the case of different shapes of nanoparticles, and tetrahedral shaped particles showed maximum efficiency for catalysis. In the case of TiO2, CeO2 and SnO2, TiO2 has minimum value of catalytic activation energy hence considered as better catalyst. Our study will find its implications in catalytic activity based applications.
Acknowledgements
One of the authors (PAB) acknowledges University Grants Commission (UGC), New Delhi for providing the financial assistance under Research Fellowship in Science for Meritorious Students (RFSMS) scheme.
References
1. Alivisatos, A.P. (1996) “Perspectives on the physical chemistry of semiconductor nanocrystals,” The Journal of Physical Chemistry, 100 (31) 13226—13239.
Publisher – Google Scholar
2. Alivisatos, A.P, Barbara, P.F, Castleman, A.W, Chang, J, Dixon, D.A, Klein, M.L, McLendon, G.L, Miller, J.S, Ratner, M.A, Rossky, P.J, Stupp, S.I. and Thompson, M.E. (1998) “From molecules to materials: current trends and future directions,” Advanced Materials, 10 (16) 1297-1336.
Publisher – Google Scholar
3. Astruc, D, Lu, F and Aranzaes, J.R. (2005) “Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis ,” Angewandte Chemie International Edition, 44 (48) 7852-7872.
Publisher – Google Scholar
4. Bell, A.T. (2003) “The impact of nanoscience on heterogeneous catalysis,” Science, 299 (5613) 1688-1691.
Publisher – Google Scholar
5. Bhatt, P.A, Pratap, A. and Jha, P.K. (2012) “Study of size-dependent glass transition and Kauzmann temperatures of tin dioxide nanoparticles,” Journal of Thermal Analysis and Calorimetry, 110 (2) 535-538.
Publisher – Google Scholar
6. Burda, C, Chen, X.B, Narayanan, R. and El-Sayed, M.A. (2005) “Chemistry and properties of nanocrystals of different shapes,” Chemical Reviews, 105 (4) 1025-1102.
Publisher – Google Scholar
7. Carrettin, S, Concepcion, P, Corma, A, Lopez Nieto, J.M. and Puntes, V.F. (2004) “Nanocrystalline CeO2 increases the activity of Au for CO Oxidation by two orders of magnitude,” Angewandte Chemie International Edition, 43 (19) 2538-2540.
Publisher – Google Scholar
8. Chen, M. and Goodman, D.W. (2006) “Catalytically active gold: From nanoparticles to ultrathin films,” Accounts of chemical research, 39 (10) 739-746.
Publisher – Google Scholar
9. Crooks, R.M, Zhao, M, Sun, L, Chechik, V. and Yeung, L.K. (2001) “Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis,” Accounts of chemical research, 34 (3) 181-190.
Publisher – Google Scholar
10. Desai, R, Gupta, S.K, Mishra, S, Jha, P.K. and Pratap, A. (2011) “The synthesis of TiO2 nanoparticles by wet-chemical method and their photoluminescence, thermal and vibrational characterizations: effect of growth condition,” International Journal of Nanoscience, 10 (6) 1249-1256.
Publisher – Google Scholar
11. Ertl, G, Knozinger, H. and Weitkamp, J. (ed.) (1997) Handbook of heterogeneous catalysis, Weinheim: Wiley-VCH.
12. Gleiter, H. (2000) “Nanostructured materials: basic concepts and microstructure,” Acta Materialia, 48 (1) 1-29.
Publisher – Google Scholar
13. Guo, M.N, Guo, C.X, Jin, L.Y, Wang, Y.J, Li, J.Q. and Luo, M.F. (2010) “Nano-sized CeO2 with extra-high surface area and its activity for CO oxidation,” Materials Letters, 64 (14) 1638-1640.
Publisher – Google Scholar
14. Gupta, S.K, Desai, R, Jha, P.K, Sahoo, S. and Kirin, D. (2010) “Titanium dioxide synthesized using titanium chloride: size effect study using Raman spectroscopy and photoluminescence,” Journal of Raman Spectroscopy, 41 (3) 350-355.
15. Gupta, S.K. and Jha, P.K. (2009) “Modified phonon confinement model for size dependent Raman shift and linewidth of silicon nanocrystals,” Solid State Communications, 149 (45-46) 1989-1992.
Publisher – Google Scholar
16. Gupta, S.K, Jha, P.K. and Arora, A.K. (2008) “Size dependent acoustic phonon dynamics of Cd Te 0.68 Se 0.32 nanoparticles in borosilicate glass,” Journal of Applied Physics, 103 (124307) 1-6.
17. Gupta, S.K, Sahoo, S, Jha, P.K, Arora, A.K. and Azhniuk, Y.M. (2009) “Observation of torsional mode in CdS1−xSex nanoparticles in a borosilicate glass,” Journal of Applied Physics, 106 (024307) 1-7.
18. Gupta, S.K, Talati, M. and Jha, P.K. (2008) “Shape and size dependent melting point temperature of nanoparticles,” Materials Science Forum, 570, 132-137.
Publisher – Google Scholar
19. Jha, P.K. (ed.) (2013) Titanium Dioxide: Applications, synthesis and toxicity, New York: Nova Science Publications.
20. Jha, P.K. and Talati, M. (2006) “Phonons in nanocrystal quantum dots,” in Chang, J.V. (ed.) Focus on Condensed Matter Physics Research, New York:Nova Science Publishers.
21. Jiang, Q. and Lu, H.M. (2008) “Size dependent interface energy and its applications,” Surface Science Reports, 63 (10) 427-464.
Publisher – Google Scholar
22. Jiang, Q, Zhang, S.H. and Li, J.C. (2004) “Grain size-dependent diffusion activation energy in nanomaterials,” Solid State Communications, 130 (9) 581-584.
Publisher – Google Scholar
23. Kung, M.C, Davis, R.J. and Kung, H.H. (2007) “Understanding Au-catalyzed low-temperature CO oxidation,” The Journal of Physical Chemistry C, 111 (32) 11767-11775.
Publisher – Google Scholar
24. Lewis, L.N. (1993) “Chemical catalysis by colloids and clusters,” Chemical Reviews, 93 (8) 2693-2730.
Publisher – Google Scholar
25. Li, G, Boerio-Goates, J, Woodfield, B.F. and Li, L. (2004) “Evidence of linear lattice expansion and covalency enhancement in rutile TiO2 nanocrystals,” Applied Physics Letters, 85 (11) 2059-2061.
Publisher – Google Scholar
26. Li, Y, Petroski, J. and El-Sayed, M.A (2000) “Activation energy of the reaction between hexacyanoferrate(III) and thiosulfate ions catalyzed by platinum nanoparticles,” The Journal of Physical Chemistry B, 104 (47) 10956-10959.
Publisher – Google Scholar
27. Lu, H.M. and Meng, X.K. (2010) “Theoretical model to calculate catalytic activation energies of platinum nanoparticles of different sizes and shapes,” The Journal of Physical Chemistry C, 114 (3) 1534-1538.
28. Mankad, V, Gupta, S.K. and Jha, P.K. (2011) “Low frequency raman scattering of anatase titanium dioxide nanocrystals,” Physica E, 44 (3) 614-617.
Publisher – Google Scholar
29. Mishra, S, Jha, P.K. and Pratap, A. (2012) “Study of size-dependent glass transition and Kauzmann temperature of titanium dioxide nanoparticles,” Journal of Thermal Analysis and Calorimetry, 107 (1) 65-68.
Publisher – Google Scholar
30. Nanda, K.K, Sahu, S.N. and Behera, S.N. (2002) “Liquid-drop model for the size-dependent melting of low-dimensional systems,” Physical Review A, 66 (013208) 1-8.
31. Narayanan, R. and El-Sayed, M.A. (2004) “Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution,” Nano Letters, 4 (7) 1343-1348.
Publisher – Google Scholar
32. Roucoux, A, Schulz, J. and Patin, H. (2002) “Reduced transition metal colloids: A novel family of reusable catalysts?,” Chemical reviews, 102 (10) 3757-3778.
Publisher – Google Scholar
33. Schmid, G. (ed.) (2005) Nanoparticles: From theory to application, Weinheim: Wiley-VCH.
34. Sharghi, H, Ebrahimpourmoghaddam, S, Memarzadeh, R. and Javadpour, S. (2013) “Tin oxide nanoparticles (NP-SnO2): preparation, characterization and their catalytic application in the Knoevenagel condensation,” Journal of the iranian chemical society, 10 (1) 141-149.
Publisher – Google Scholar
35. Somorjai, G.A, Contreras, A.M, Montano, M. and Rioux, R.M. (2006) “Clusters, surfaces, and catalysis,” Proceedings of The National Academy of Sciences of the United States of America, 103 (28) 10577-10583.
Publisher – Google Scholar
36. Sun, C.Q. (2007) “Size dependence of nanostructures: Impact of bond order deficiency,” Progress in Solid State Chemistry, 35 (1) 1-159.
Publisher – Google Scholar
37. Talati, M. and Jha, P.K. (2005) “Low-frequency acoustic phonons in nanometric CeO2 particles,” Physica E, 28 (2) 171-177.
Publisher – Google Scholar
38. Wasylenko, W. and Frei, H. (2007) “ Direct observation of the kinetically relevant site of CO hydrogenation on supported Ru catalyst at 700 K by time-resolved FT-IR spectroscopy,“ Physical Chemistry Chemical Physics, 9 (40) 5497-5502.
Publisher – Google Scholar
39. Yang, C.C, Armellin, J. and Li, S. (2008) “Determinants of thermal conductivity and diffusivity in nanostructural semiconductors,” The Journal of Physical Chemistry B, 112 (5) 1482-1486.
Publisher – Google Scholar
40. Yang, C.C. and Jiang, Q. (2006) “Size effect on the bandgap of II—VI semiconductor nanocrystals,” Materials Science and Engineering B, 131 (1-3) 191-194.
Publisher – Google Scholar
41. Yang, C.C. and Li, S. (2008) “Size-dependent raman red shifts of semiconductor nanocrystals,” The Journal of Physical Chemistry B, 112 (45) 14193-14197.
Publisher – Google Scholar
42. Zhang, Z, Li, J.C. and Jiang, Q. (2000) “Modeling for size-dependent and dimension-dependent melting of nanocrystals,” Journal of Physics D: Applied Physics, 33 (20) 2653-2656.
43. Zhou, X, Xu, W, Liu, G, Panda, D. and Chen, P. (2010) “Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level,” Journal of the American Chemical Society, 132 (1) 138-146.