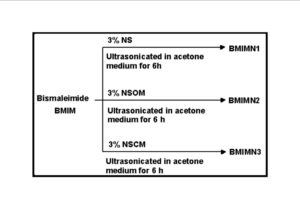
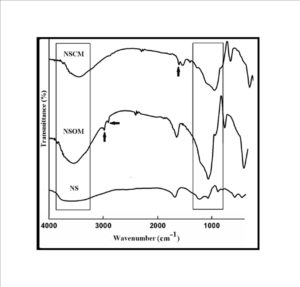
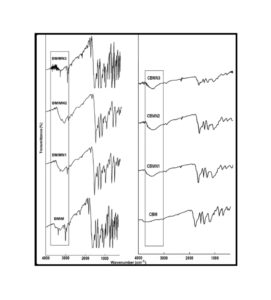
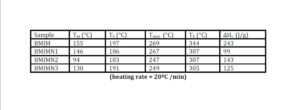
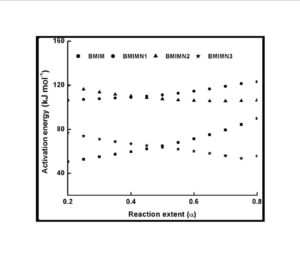
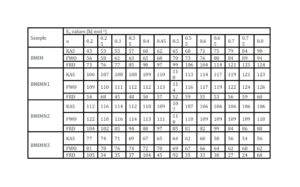
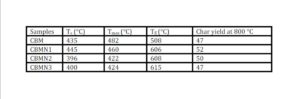
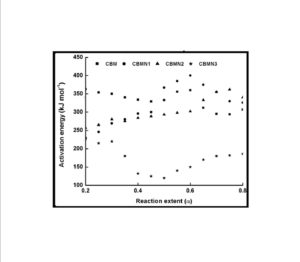
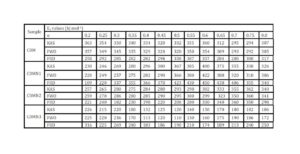
The trend in the activation energy variation noted for the degradation of the material CBMN1 is very similar to that of CBM but the activation energy at the initial stage is lower than the CBM but after α = 0.40, the activation energy value is 30-40 kJ mol-1 higher than CBM. At the initial stage the nanosilica particles may act as medium for thermal transport for the degrading material, thus requiring lower activation energy for degradation. The increase in activation energy value after α = 0.40 may be attributed to the shielding effect of nanoparticle in the evolution of formed gases from polymer matrix, during its thermal decomposition [41].
CBMN2 also shows similar trend as that of CBMN1, but its activation energy values are lower than CBM and CBMN1 up to α = 0.60, but afterwards the value reaches a higher region compared to both the systems. At the initial stage the catalytic activity exhibited by NSOM particle makes the degradation of CBMN2 as an easier one; as a result the Ea values are lower. Further, the degradation of the long alkyl chains present in the NSOM particles may also add to this factor. But after 0.60 reaction extent value the Ea value get increased this may due to the restriction of mobility of the segmental movement of degrading chains exhibited by the enhanced interaction between the silica and the polymer matrix.
The trend shown by CBMN3 is completely different from the other three systems. The activation energy values are present in the lower region. In the chemically modified nanosilica particles incorporated BMIM system, the maleimide units chemically bound with the silica units is having the possibility to polymerize with the maleimide double bonds present in the BMIM monomer. The chemically modified silica particles act as spacers and this bulky spacer invariably reduces the crosslinking density in the cured BMIM (CBMN3). Due to this aspect, during the degradation there will be enhanced motion of the chain segments favoring easy degradation [42].
Conclusions
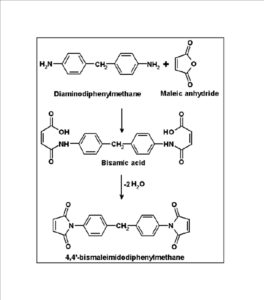
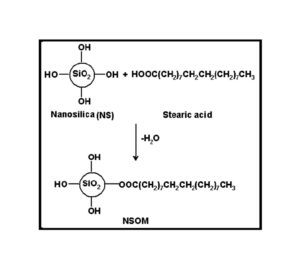
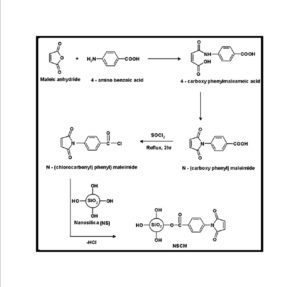
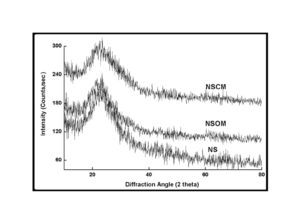
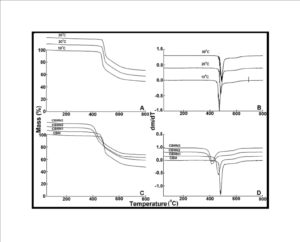
BMIM was prepared by the classic amic acid approach. Nanosilica particles were obtained in a very economical way from the largest available agricultural waste rice husk, and organic and chemical modifications were done in the nanosilica particles. The interaction existing between the nanosilica particles and BMIM was confirmed by FTIR studies. The curing studies of BMIM and its nanosilica blends were done by DSC analysis. Nanosilica and modified nanosilica particles decrease the melting point, curing temperatures and enthalpy of curing of BMIM. This decrease in curing parameters may be due to the catalytic activity exhibited by the NS and modified NS particles, which can accelerate the curing process. The degradation temperatures of BMIM/nanosilica nanocomposites determined by TGA were lower than that of pure polyBMIM matrix. The thermal degradation products produced from the modified NS particles induced catalytic activity on the thermal degradation of polyBMIM matrix. This leads to decrease in the degradation temperature. The curing kinetics of BMIM and its nanosilica blends and the degradation kinetics of polyBMIM and its nanosilica nanocomposites were calculated using three model free kinetic methods KAS, FWO and FRD methods. The Ea values for the curing of NS reinforced BMIM increased as the extent of polymerization increased, but NSOM and NSCM reinforced BMIM show higher Ea values at the initial stages of curing but as the curing proceeds the Ea values decreased because of the catalytic activity exhibited by NSOM and NSCM particles. The trend in the variation of Ea values for the degradation of BMIM/nanosilica nanocomposites was more or less similar up to the reaction extent value 0.45.
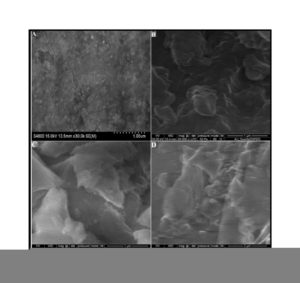
Afterwards, NS, NSOM particles restrict the molecular segmental movement of polyBMIM, leading the increase in Ea values. For NSCM reinforced system, the Ea values decreased because of physical and chemical changes took place in the chemical moieties present it. The SEM images of modified nanosilica particles reinforced BMIM matrix show broken surfaces and rough fractured strips indicating the improvement in the toughness of the nanocomposites.
Acknowledgements
The authors wish to express sincere thanks to the management and principal of the respective colleges for providing all facilities to do the work. The authors would like to thank the Directorate of Extramural Research & Intellectual Property Rights (ER&IPR), Defence Research & Development Organization, Ministry of Defence, Government of India, New Delhi-110 105 for financially supporting this work under the grant ERIP/ER/0704359/M/01/1101 dated 12-12-2008.
References
1. Niasari,M.S. and Ghanbari,D. (2009) ‘Polymeric nanaocompoaite materials,’ Inorganic Chimica Acta, 10(3), 351- 362.
2. Vlasveld,D.P.N., Parlevliet,P.P., Berse,H.E.N. and Picken,S.J. (2005) ‘Fibre-matrix adhesion in glass-fibre reinforced polyamide-6 silicate nanocomposites,’ Composites, 36 (1), 1-11.
3. Legashetty, A.and Venkatraman, A. (2005) ‘Polymer nanocomposites,’ Resonance, 10 (7), 49-60.
4. Azeredo, H.M.C., Mattoso, L.H.C. and McHugh, T.H. (2009) ‘Nanocomposite edible films from mango puree reinforced with cellulose nanofibers,’ Journal of Food Science, 74 (5), N31-N35.
5. Hussain, F., Hojjati, M., Okamoto, M. and Gorga, R.E. (2006) ‘Review article: Polymer-matrix nanocomposites, processing, manufacturing and application: An overview,’ Journal of Composite Materials, 40 (17), 1511-1575.
6. Sarkawi, S.S. and Aziz, Y. (2003) ‘Ground rice husk as filler in rubber compounding,’ Journa Teknologi, 39(A), 135-148.
7. Habeeb, G.A. and Mahmud, H.B. (2010) ‘Study on properties of rice husk ash and its use as cement replacement material,’ Materials research, 13 (2), 185-190.
8. Yalcin, N. and Sevind, V. (2001) ‘Studies on silica obtained from rice husk,’ Ceramics International, 27 (2), 219-224.
9. De Sousa, A.M., Visconte, L., Mansur, C. and Furtado, C. (2009) ‘Silica sol obtained from rice husk ash,’ Chemical Techonology, 3(4), 371.
10. Sivaprasad, D. and Ramakrishna, A. (2010) ‘Fabrication and characterization of A356.2-rice husk ash composite using stir casting technique,’ Interanational Journal of Engineering Science and Technology, 2 (12), 7603-7608.
11. Tang, J.C., Yang, H.C., Chen, S.Y. and Chen-Yang, Y.W. (2007) ‘Preparation and properties of polyimide/silica hybrid nanocmoposites,’ Polymer Composites, 28(5), 575-581.
12. Chrissafis, K. (2009) ‘Kinetics of thermal degradation of polymers-complementary use of isoconversional and model fitting methods,’ J. Therm. Anal. Calorim., 95 (1), 273-283.
13. Kissinger, H.E. (1957) ‘Reaction kinetics in differential thermal analysis,’ Analytical Chemistry, 29(11), 1702-1706.
14. Akahira, T. and Sunose, T. (1971) ‘Method of determining activation deterioration constant of electrical insulating materials,’ Res.Report, Chiba.Inst.Technol. (Sci.Technol.), 16, 22-31.
15. Ozawa, T. (1965) ‘A new method of analyzing thermogravimetric data,’ Bull. of the Chemical Society of Japan, 38(11), 1881-1886.
16. Flynn J.H. and Wall, L.A. (1966) ‘A quick, direct method for the determination of activation energy from thermogravimetric data,’ Polymer Letters, 4(5), 323-328.
17. Flynn, J.H. and Wall, L.A. (1966) ‘General treatment of the thermogravimetry of polymers,’ Journal of Research of the National Bureau of Standards Physical Chemistry, 70A(6), 487-523.
18. Friedman, H.L. (1964). ‘Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic,’ Journal of Polymer Science Part C-Polym.Symp., 6(1), 183-195.
19. Yao, F., Wu, Q., Lei, Y., Guo, W. and Xu, Y. (2008) ‘Thermal decomposition kinetics of natural fibres: Activation energy with dynamic thermogravimetric analysis,’ Polymer Degradation Stability, 93(1), 90-98.
20. Thuadij, N. and Nuntiya, A. (2008) ‘Preparation of nanosilica powder from rice husk ash by precipitation method,’ Chiang Mai.Journal of Science, 35(1), 206-211.
21. Awizar, D.A., Othman, N.K., Jalar, A., Daud, A.R., Rahman I.A. and Harden, N.H.A. (2013) ‘Nanosilicate extraction from rice husk ash as green corrosion inhibitor,’ International Journal of Electrochemical Science, 8(2), 1759-1769.
22. Rafiee, E., Shahebrahimi, S., Feyzi, M. and Shaterzadeh, M. (2012) ‘Optimization of synthesis and characterization of nanosilica produced from rice husk ( a common waste material),’ International Nanoletters, 2(29), 1-5.
23. Theivasanthi, T. and Alagar, M. (2010) ‘X-ray diffraction studies of copper nanopowder,’ Archives of Physics Research, 1(2), 112-117.
24. Liu, W., Wu, L., Tian, X., Zheng, J., Cui, P., He, A. and Zhu, C. (2010) ‘Morphology studies of poly(ethylene terephthalate)/ silica nanocomposites,’ Polymer Bulletin, 65(2), 133-143.
25. Hussain, M.F., Chen, J. and Hojjati, M. (2007) ‘Epoxy-silicate nanocomposites: Cure monitoring and characterization,’ Material Science and Engineering-A, 445-446, 467-476.
26. Surender, R., Mahendran, A., Thamaraichelvan, A., Alam, S. and VIjayakumar, C.T. (2013) ‘Curing studies of bisphenol a based bismaleimide and cloisite 15a nanoclay blends using differential scanning calorimetry and model-free kinetics,’ Journal of Applied Polymer Science, 128 (1), 712-724.
27. Surender, R., Mahendran, A.R., Alam, S. and Vijayakumar, C.T. (2013) ‘Indane-based bismaleimide and cloisite 15a nanoclay blends: Kinetics of thermal curing and degradation of particulate nanocomposites,’ Polymer Composites, 34(8), 1279-1297.
28. Ghaemy, M. Bazzar, M. and Mighani, H. (2011) ‘Effect of nanosilica on the kinetics of cure reaction and thermal degradation of epoxy resin,’ Chinese Journal of Polymer Science, 29 (2), 141-148.
29. Gabilondo, N., Lopez, M., Ramos, J.A., Echeverria, J.M. and Mondragon, I. (2007) ‘Curing kinetics of amine and sodium hydroxide catalyzed phenol-formaldehyde resins,’ Journal of Thermal Analysis and Calorimetry, 90(1), 229-236.
30. Guigo, N., Mija, A., Vincent L. and Sbirrazzuble, N. (2007) ‘Chemorheological analysis and model-free kinetics of acid catalysed furfuryl alcohol polymerization,’ Physical Chemistry Chemical Physics, 9(39), 5359-5366.
31. Jiasheng, Q. and Pingsheng, H.E. (2003) ‘Non-isothermal crystallization of HDPE/nano-SiO2 composite,’ Journal of Materials Science, 38(11), 2299-2304.
32. Sheng, X., Akinc. M. and Kessler, M.R. (2008) ‘Cure kinetics of thermosetting bisphenol E cyanate ester,’ Journal of Thermal Analysis and Calorimetry, 93(1), 77-85.
33. Satheesh Chandran, M., Krishna, M., Rai, S., Krupashankara M.S. and Salini, K. (2012). ‘Cure Kinetics and activation energy studies of modified bismaleimide resins,’ ISRN Polymer Science, 2012, 13-21.
34. Zhou, T., Wang, X., H.Liu, X., and Lai, J.Z. (2010) ‘Effect of silane treatment of carboxylic-functionalized multi-walled carbon nanotubes on the thermal properties of epoxy nanocomposites,’ eXPRESS Polyme Latters, 4 (4), 217-226.
35. Rajamani, D., Surender, R., Alam, S., Muthusubramanian S. and Vijayakumar, C.T. (2013) ‘Bismaleimide/rice husk silica blends-kinetics of curing by DSC,’ Science Jet, 2, 40-44.
36. Surender, R., Mahendran, A., Thamaraichelvan, A., Alam S. and Vijayakumar, C.T. (2013) ‘Model free kinetics-Thermal degradation of bisphenol A based polybismaleimide-closite 15a nanocomposites,’ Thermochimca Acta, 562(20), 11-21.
37. Vijayakumar, C.T., Thenmozhi, K., Surender, R., Sivasamy, P. and Alam, S. (2012) ‘Degradation kinetics of bismaleimide – natural clay composites,’ Science Jet, 17, 1-6.
38. Sivasamy, P., Meenakshisundaram, M. and Vijayakumar, C.T. (2003) ‘A study on the effect of para substitution on the thermal degradation of poly N-arylmaleimides,’ Journal of Analytical and Applied Pyrrolysis, 68-69, 51-59.
39. Torrecillas, R., Regnier, N. and Mortaigne, B. (1996) ‘Thermal degradation of bismaleimide and bisnadimide networks – products of thermal degradation and type of crosslinking points,’ Polymer Degradation and Stability, 51(3), 307-318.
40. Xie, W., Pan, W.P. and Chung, K.C. (2001) ‘Thermal Characterization of PMR polyimides,’ Thermochimca Acta, 367 -368, 143-153.
41. Chrissafis, K., Paraskevopoulos, K.M., Pavlidou, E. and Bikiaris, D. (2009) ‘Thermal degradation mechanism of HDPE nanocomposites containing fumed silica nanoparticles,’ Thermochimica Acta, 485(1), 65-71.
42. Rajamani, D., Surender, R., Mahendran, A., Muthusubramanian S. and Vijayakumar, C.T. (2013) ‘Bismaleimide rice husk silica reinforced composites: Synthesis and thermal degradation studies,’ Journal of Thermal Analysis and Calorimetry, 114(2), 883-893 .