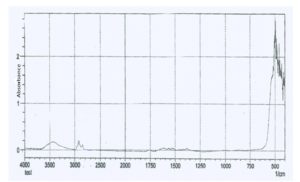
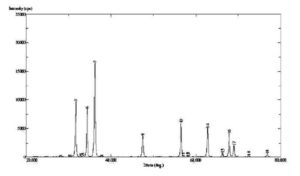
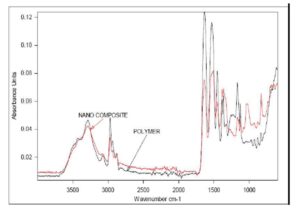
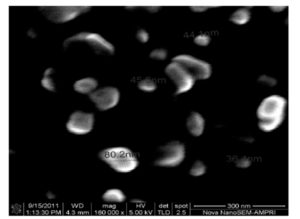
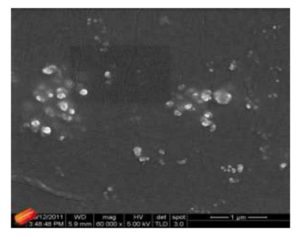
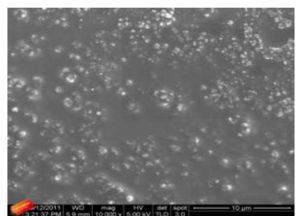
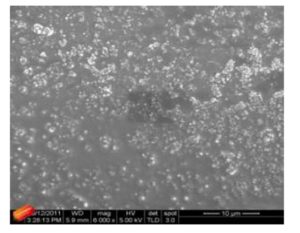
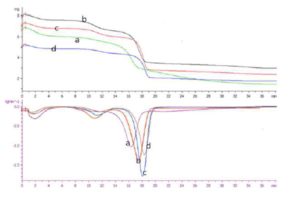
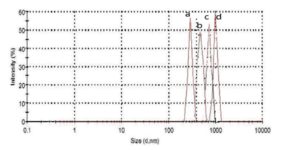
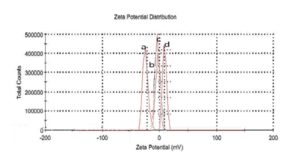
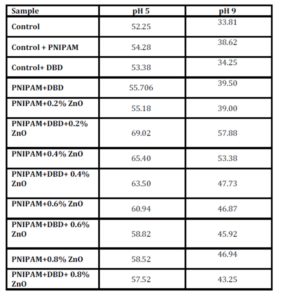
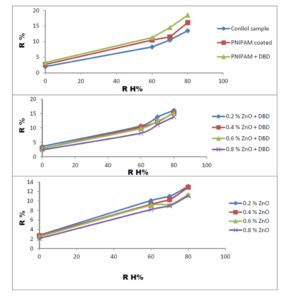
With this new procedure, we have created a tool to obtain a broad range of new inorganic organic hybrid materials in a very easy way for textile applications.
Acknowledgement
The authors are grateful to Department of Science and Technology (DST), India and Fundação para a Ciência e a Tecnologia (FCT), Portugal for the financial support to this study.
References
1. Arnold, MS., Avouri, P., Pan, ZW. and Wang, ZL (2003)’Field- Effect transistors based on single semiconducting oxide nanobelts,’ J Phys Chem, B 107, 659-663.
Publisher – GoogleScholar
2. Baglioni, PL., Dei, L., Fratoni, P., Lo Nostro, M. and Moroni, M.(2003) ‘Preparation of nano- and micro-particles of group II and transition metals oxides and hydroxides and their used in the ceramic, textile and paper industries,’Patent WO 2003082742
3. Fei, B., Deng, Z., Xin,JH., Zhang, Y. and Pang, G. (2006) ‘Room temperature synthesis of rutile nanorods and their applications on cloth,’Nanotechnology, 17, 1927-1931.
Publisher – GoogleScholar
4. Fu, Q., Rama Rao, G., Ward., V, Lu, T.L. and Lopez, G. P. (2007). ‘Thermoresponsive transport through ordered mesoporous silica/PNIPAAm copolymer membranes and microspheres,’ Langmuir, Vol. 23, 170-174 ISSN: 0743-7463.
GoogleScholar
5. Gil, SS., Gyu, L. and Jin-Hae, C.(2003) ‘Synthesis and LCST behavior of thermoresponsive poly(N- iso propyl acrylamide) — clay nanocomposites,’ J. Ind. Eng. Chem., 9 (1 ), 58 — 62.
6. Grancariæ, AM., Markoviæ, L. and Tarbuk, A. (2007) ‘Active multifunctional cotton treated with zeolite nanoparticles,’ Tekstil, 56, 543-553.
GoogleScholar
7. Grancariæ, AM., Tarbuk, A., Dumitrescu, I. and Bišæan, J. (2006) ‘UV protection of pretreated cotton – influence of fwa’sfluorescence,’ AATCC Review, 6, 40-46.
8. Grancariæ, AM. and Tarbuk, A. (2009) ‘EDA modified pet fabric treated with activated natural zeolite nanoparticles,’Materials Technology, 24, 58-63.
Publisher – GoogleScholar
9. Ibrahim, NA., Eid, BM., Abd El-Aziz, E. and Abou Elmaaty T.M. (2013) ‘ Functionalization of Linen/Cotton Pigment Prints Using Inorganic Nano Structure Materials’, Carbohydrate Polymers, 97, 537-545.
Publisher – GoogleScholar
10. Ibrahim, NA., Eid, BM. and El-Batal,H. (2012) ‘A Novel Approach for Adding Smart Functionalities to Cellulosic Fabrics’, Carbohydrate Polymers, 87, 744-751.
Publisher – GoogleScholar
11. Ibrahim, NA., Refaie, R. and Ahmed,A.F. (2010) ‘Novel Approach for Attaining Cotton Fabric with Multi Functional Properties’, Journal of Industrial Textiles, 40 (1), 65-83.
Publisher – GoogleScholar
12. Lee, HJ., Yeo, SY. and Jeong, H. (2003) ‘Antibacterial effect of nanosized silver colloidal solution on textile fabrics,’ J Mater Sci., 38, 2199-2204.
Publisher – GoogleScholar
13. Liu, B. and Hu, J. (2005) ‘The application of temperature-sensitive hydrogels,’ Fibres & Textiles in Eastern Europe, 13(6), 45-49.
GoogleScholar
14. Pan, YV., Wesley, RA., Luginbuhl, R., Denton, DD. and Ratner, B.D. (2001`) ‘Plasmapolymerized N-isopropylacrylamide: Synthesis and characterization of a smart thermally responsive coating ,’Biomacromolecules, 2, 32-36.
Publisher – GoogleScholar
15. Pan, ZW., Dai, Z.R. and Wang, Z.L. (2001) ‘Nanobelts of semiconducting oxides,’ Science, 291, 1947-1949.
Publisher – GoogleScholar
16. Qi, K., Chen, X., Liu, Y., Xin, JH., Mak, CL. and Daoud, W.A. (2007) ‘Facile preparation of anatase/SiO2 spherical nanocomposites and their application in self cleaning textiles,’J Mater Chem., 17, 3504-3508.
Publisher – GoogleScholar
17. Riva, AI., Algaba, M. and Pepio, M. (2006) ‘Action of a finishing product in the improvement of the ultraviolet protection provided by cotton fabrics. Modelisation of the effect Cellulose, 13 , 697-704
Publisher – GoogleScholar
18. Sawai, J. (2003) ‘Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay,’ J Microbiol Methods, 54, 177-182.
Publisher – GoogleScholar
19. Scalia, SR., Tursilli, A., Bianchi, P., Lo Nostro, Bocci, E., Ridi, F. and Baglioni, P. (2006) ‘Incorporation of the sunscreen agent, octyl methocycinnamate in a cellulosic fabric grafted withb-cyclodextrin,’ Int J Pharm, 308, 155-159.
Publisher – GoogleScholar
20. Schild, HG. (1992) ‘Poly(N-isopropylacrylamide): experiment, theory and application,’ Prog. Polym. Sci., 17, 163-249.
Publisher – GoogleScholar
21. Sorna Gowri, V., Luís , A., Maria Teresa, PA., Noémia, CP., Pedro Souto, A., Maria Fátima, E. and Sanghi , SK. (2010) ‘Functional finishing of polyamide fabrics using ZnO—PMMA Nanocomposites’, Journal of Materials Science, 45, 2427-2435.
Publisher – GoogleScholar
22. Suzuki, K., Yumura, T., Mizuguchi, M., Tanaka, Y., Chen, C.W. and Akashi, M.(2000) ‘Poly(N-isopropylacrylamide)-grafted silica as a support of platinum colloids: Preparation method, characterization, and catalytic properties in hydrogenation ,’J.Applied Polymer Sci. , 77, 2678-2684.
Publisher – GoogleScholar
23. Tang, E., Cheng, G., Pang, X., Ma, X. and Xing, F. (2006) ‘Synthesis of nano- ZnO/poly(methylmethacrylate) composite microsphere through emulsion polymerization and its UV shielding property,’ Colloid Polym Sci, 284, 422- 428.
Publisher – GoogleScholar
24. Turkoglu, MS. and Yener, S. (1997) ‘Design and in vivo evaluation of ultrafine inorganic-oxide-containing-sunscreen formulations,’ Int J Cosmet Sci ,19, 193-201.
Publisher – GoogleScholar
25. Vigneshwaran, N., Kumar S., Kathe, AA., Varadarajan, PV. and Prasad, V. (2006) ‘Functional finishing of cotton fabrics using zinc oxide-soluble starch nanocomposites,’ Nanotechnology, 17(20), 5087-5095.
Publisher – GoogleScholar
26. Wang, R.H., Xin, J.H. and Tao, X.M. (2005) ‘UV-blocking property of dumbbell- shaped ZnO crystallites on cotton fabric,’ Inorg Chem, 44(11), 3926-3930.
Publisher – GoogleScholar
27. Wang, R.H, Xin, J.H., Tao, X.M. and Daoud, W.A. (2004) ‘ZnO nanorods grown on cotton fabrics at low temperature,’ Chem Phys Lett, 398(18), 250-255.
Publisher – GoogleScholar
28. Xin, J.H., Daoud, W.A. and Kong, Y.Y .(2004) ‘A new approach to UV-blocking treatment for cotton fabrics,’ Text Res J., 74(2), 97-10.
Publisher – GoogleScholar
29. Xiong, M., Gu, G., You, B. and Wu, L. (2003) ‘Preparation and characterization of poly(styrene butylacrylate) latex/nano-ZnO nanocomposites,’ J Appl Polym Sci., 90(7), 1923-1931.
Publisher – GoogleScholar
30. Yadav, A., Prasad, V., Kathe, A.A., Raj, S., Yadav, D., Sundaramoorthy, C. and Vigneshwaran, N. (2006) ‘Functional finishing in cotton fabrics using zinc oxide nanoparticles,’ Bull Mater Sci ,29(6), 641-645.
Publisher – GoogleScholar