Located in a tropical belt, India is endowed with a rich wealth of fauna and flora with inumerous medicinal plants. These plants have made a good contribution to the development of ancient Indian Materia Medica. One of the earliest treatises on Indian medicine, the Charaka Samhita (1000 B.C), records the use of over 340 drugs of vegetable origin (Pullaiah, 2006). Most of these continue to be gathered from wild plants to meet the demand of the medical profession. India, in particular, has a big scope for the development of the pharmaceutical and phytochemical industry. The Indian Pharmacopoeia (1966) recognizes 85 drug plants whose ingredients are used in various pharmaceutical preparations. During the last two decades, the pharmaceutical industry has made massive investments on pharmacological, clinical and chemical researches all over the world in an effort to discover still more potent plant drugs; in fact, a few new drug plants have successfully passed the tests of commercial screening.
Herbs have been shown to contain varied levels of phytochemicals like polyphenols (Bravo, 1998; Chung et al., 1998; Crozier, 2000; Urquiaga and Leighton, 2000), tannins (Mekhfi, 2006; Chen et al., 2009), flavonoids (Hertog et al., 1993; Percival, 1998; Urquiaga and Leighton, 2000), lignins (Dizhbite et al., 2004; Ralph et al., 2007; Garcia et al., 2010), alkaloids (Sawant et al., 2004; Zhang et al., 2007; Staerk et al., 2009), glycosides (Yokosuka et al., 2009; Leong et al., 2010), phytosterols (Amsterdam et al., 2005), essential oils (Oliveira et al., 2010; Tohidpour et al., 2010) and saponins (Cheeke, 1998; Chen et al., 2009) which have various pharmaceutical properties. Among them antioxidants (Dickinson, 2002; Pouteau et al., 2003; Adedapo et al., 2008), antimicrobial compounds (Basim et al, 2005; Adeyemi et al, 2008; Ghalem and Mohamed, 2009), anticancer agents (Weng et al., 2009; Silva et al., 2008; Yokosuka et al., 2009), antiageing molecules (Turner, 2006), agents that prevent cardiovascular disease and many other health promoting chemicals are importantly being studied in herbal plants.
In recent years there has been a remarkable increment in scientific articles dealing with oxidative stress. To counter the oxidative stress, herbal products give promise as a potential treatment. Therefore identification of flavonoids and other dietary polyphenol antioxidants present in plant foods as bioactive molecules is imperative. The present data available in the literature also supports the idea that health benefits associated with fruits, vegetables and red wine in the diet are probably linked to the polyphenol antioxidants they contain. Tree bark extracts contain many antioxidants that can help neutralize free radicals in everyday life by boosting the immune system.
The use of herbal drugs to treat infectious disease has become popular worldwide now. Infectious diseases represent a critical problem to health and they are one of the main causes of morbidity and mortality worldwide. The development of antibiotic resistant bacteria and the toxicity caused by prolonged drug treatment has led to the wide use of medicinal plants by the traditional medical practitioners for curing various diseases in their day to day practice (Kaur et al., 2009). Indians have long been using many of the herbal products and formulations to counter the microbial activity on open wounds and skin infections well before the inventions of antibiotics. A wide range of medicinal plants have antibacterial and antifungal activity as evidenced by the ethno botanical use besides being strengthened by the scientific data. Nowadays, bacteria still threaten human life even though the occurrence of many antibiotics has improved human health in the past eighty years. For the past decade or more multiple drug resistant strains of bacteria such as methicillin resistant Staphylococcus aureus (MRSA), Enterococci and other Gram positive cocci have been highlighted and showcased in the medicine (Tohidpour et al., 2010).
A lot of plants with medicinal value used in the Indian traditional medicine have not yet been characterized for their active principles. Hence, these plants remain unrecognized for their potential uses. Research on such plants can provide useful information for their exploitation in treating diseases. One such plant of great medicinal importance is P. longifolia which is distributed in the Western Ghats. The products of this plant have been used widely in Ayurveda for treating arthritis; stomach disorders wound healing and now prescribed for obesity by local folklore practitioners. However, proper scientific information on the medicinal properties of the plant has not been taken up by any researchers.
The objectives of the present investigation were to quantify the total phenolics and antioxidant activities in the ethanolic, methanolic, aqueous, and ethyl acetate extracts of – P. longifolia and to study the antimicrobial activities of different extracts on various bacterial and fungal species.
Material and Methods
Plant material collection: The plant Pajanelia longifolia was identified and verified by a taxonomist and the herbarium is deposited in the department. The bark was collected and air dried. Coarse powder of the dried bark of P. longifolia was prepared by milling the same in Alva’s Pharmacy, Mijar, Moodbidri. The powder was stored at dark, warm and dry place in the laboratory.
Preparation of extracts: Water, 70% methanol, ethanol and ethyl acetate extracts were chosen for the present study and accordingly the powdered barks of P. longifolia was extracted with various solvents by soxhlet method and concentrated by using rotary evaporator. The extracts were stored at -10oC. For each type of solvent extraction three replicates were taken.
Determination of total phenolics: The amount of phenolic compound in the extracts of P. longifolia was determined using the Folin-Ciocalteau (FC) assay using pyrocatechol as a standard (Sadasivam and Manikam, 2008). 25 mg of the extract was dissolved in 5 ml of respective solvent and diluted to 50 ml with distilled water in a standard volumetric flask. Exactly 1 ml sample of three different concentrations (50 µg, 100 µg and 250 µg) of the extract were treated with 0.5 ml of Folin-Ciocalteau reagent and the reaction mixture was incubated for 3 minutes at room temperature. To this 2 ml of 20% Na2CO3 was added and the tubes were kept in a boiling water bath for exactly 1 minute. The tubes were allowed to cool. The absorbance was measured against a respective solvent blank at 650nm using a UV-visible spectrophotometer (Elico, SL159).
Determination of antioxidant activity: The Ferric Reducing Antioxidizing Power assay (FRAP assay) was used to determine the antioxidizing activity of the extracts. A modified method of Adedapo et al., (2008) was adopted. The stock solutions included 300 mM acetate buffer (3.1 g C2H3NaO2-3H2O and 16ml C2H4O2), pH 3.6, 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3-6H2O solution. The fresh working solution was prepared by mixing 25ml acetate buffer, 2.5 ml TPTZ, and 2.5 ml FeCl3-6H2O.
Determination of antioxidant activity: Four different concentrations 50 µg, 100 µg, 175 µg and 250 µg of the water extract, 70% methanol extract, ethanol and ethyl acetate extracts were prepared. Exactly 1 ml of each sample concentration was treated with 3 ml FRAP reagent and the absorbance was read at 593 nm immediately in a UV-visible spectrophotometer (Elico, SL159). Iron (II) sulfate (FeSO4-7H2O) was used as the standard. The antioxidant activity of the extracts was also compared with standard ascorbic acid (200µM to 1000µM).
Determination of Antibacterial Activity:
Test organisms: The test organisms used for the study are Staphylococcus aureus, Escherichia coli, Ballicus subtilis, Proteus mirabilis, Vibrio parahaemolyticus., Lactobacillus casei and L. fermentum. The microorganisms were taken from the laboratory collection kept at the Department of Biotechnology, Alva’s College, Moodbidri. The isolates were subcultured in Mueller-Hinton broth and were maintained as slants of the same agar medium.
Well diffusion assay: 24 hour old culture of each of the above mentioned test bacterium grown in Mueller—Hinton broth, was swabbed over the Mueller—Hinton agar plates with a sterile cotton swab. Six wells were bored into each swabbed plate. As much as 120 µL of each extract of different concentrations 500 µg, 1000 µg, 2000 µg and 5000 µg was added to four wells per plate. Penicillin served as the positive control and 50% DMSO was added which served as the negative control. The plates were incubated at 37oC for 24 hours. Diameters of the inhibition zones were measured in millimeters (mm).
Determination of Antifungal Activity:
Test organisms: Bread mould fungi were isolated and cultured in Sabouraud’s Dextrose broth at room temperature for 48 hours.
Well diffusion assay: Sabouraud’s dextrose agar was used for well diffusion assay of fungal cultures. 48 hour old culture of each fungal culture in Sabouraud’s dextrose broth was swabbed over the Sabouraud’s dextrose agar plates with a sterile cotton swab. Six wells were bored into each swabbed plate. As much as 120 µL of each extract of different concentrations 500 µg, 1000 µg, 2000 µg and 5000 µg was added to four wells per plate. To one well Nystatin was added which served as the positive control and to the remaining well 50% DMSO was added which served as the negative control. The plates were incubated at room temperature (28oC) for 48 hours. After 48 hours, the plates were observed for inhibition zones and the diameters of the inhibition zones were measured in millimeter (mm).
Results and Discussion
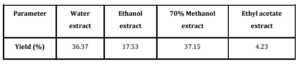
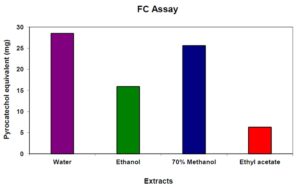
Percentage yield of various bark extracts of Pajanelia longifolia in different solvent system was given in table 1. Water extract of bark powder of the plant yielded a final product of 36.37%. Similarly, a high percentage of 37.15 was recorded for methanol extract followed by 17.53 and 4.23 for ethanol and ethyl acetate extract respectively. The level of phenolic compounds in the plant extracts is considerable. The highest phenolic content of 28.51 mg pyrocatechol equivalent/g raw material was observed in the water extract. On the other hand, the ethyl acetate extract showed least phenolics content of 6.31 mg pyrocatechol equivalent/g raw material. In case of 70% methanol extract and ethanolic extract the total polyphenols amounted to 25.62 and 15.94 mg pyrocatechol equivalent/g raw material respectively (Fig. 1). Phenol and phenolic compound such as flavonoids have been shown to possess significant antioxidant activities. Aiyegoro et al., (2009) estimated polyphenol content of the aqueous extract of Helichrysum pedunculatum leaves by FC method and they found it to be 0.512 mg gallic acid equivalent/g of extract. Adedapo et al., (2008) reported that methanol extract of the stem of Calpurnia aurea has higher total phenolics (11.79 mg tannic acid/g of dry plant material) then that of the leaf extract (9.62 mg tannic acid/g of dry plant material). Amarowicz et al., (2009) have reported polyphenol content of 290 mg/g in fractions of red lentil extract. In the present study also, water and methanolic extract showed the higher polyphenols and the results are supported with the earlier reports (Bravo, 1998; Chung et al., 1998; Crozier et al., 2000). Polyphenols exhibit a wide range of biological effects as a consequence of their antioxidant properties (Adedapo et al., 2008). They inhibit LDL oxidation in vitro (Frankel et al., 1993). Polyphenols have been also found to protect DNA from oxidative damage with important consequences in the age related development of some cancers (Halliwell, 1999). In addition, polyphenols have also shown antithrombotic and anti-inflammatory effect (Gerritsen et al., 1995; Mulddon and Kritchevsky, 1996).
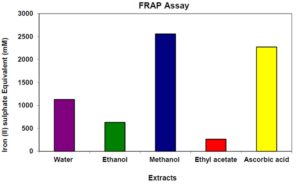
The total antioxidant activity of the bark extracts was found to be in the range of 250 to 2600 mM Fe (II)/g raw material as showed in figure 2. Among the four extracts, 70% methanol extract showed the highest antioxidant activity (2566 mM Fe (II)/g raw material). Standard ascorbic acid showed an antioxidant activity of 2272 mM Fe (II)/g standard ascorbic acid. However, the Ethyl acetate extract had the lowest (262 mM Fe (II)/g raw material) antioxidising activity. In case of water extract and ethanol extract the antioxidant activity showed 1127 and 630 mM Fe (II)/g raw material respectively. Antioxidant activity increased proportionally with the polyphenol content. Zargar et al., (2011) in their study showed that methanol extract of Vitex negundo leaf exhibited significantly (p < 0.05) higher antioxidant activity in terms of measurements of DPPH free radical (IC50), FRAP and β-carotene-linoleic assays than those of hexane extract and essential oil. Methanol extract of V. negundo leaf also contained high amounts of bioactive compounds including total phenolic compounds (363 mg GAE/g), epicatechin (16.98 mg/g), quercetin (13.45 mg/g), catechin (8.95 mg/g) and myricetin (3.32 mg/g) while the concentrations of tocopherol, β-carotene and lycopene were found to be lower.
According to recent reports, a highly positive relationship between total phenols and antioxidant activity appears to be the trend in many plant species (Adedapo et al., 2008; Souri et al., 2008). Kumar et al., (2010) found that methanol leaf extract of Koelreutaria paniculata, Acacia catechu and Mimusops hexandra showed strong inhibitory activity whereas that of Hamelia patens exhibited moderate DPPH radical scavenging activity at concentration of 200 μg/ml. However, methanol extract of Swietenia mahogoni, Murraya exotica, Murraya koenigii, Alstonia scholaris, Ficus benjamina and Sapindus trifoliatus exhibited weak hydrogen donating potential in DPPH assay. Sivakumar et al., (2010) observed the total phenolic and total tannins were quantitatively estimated in stem parts of Tinospora cordifolia in 7.2 % w/w and 8.7 % w/w respectively. They concluded that the greater amount of phenolic and tannins compounds leads to more powerful free radical scavenging effect as shown by methanolic extract of Tinospora cordifolia stem. Adedapo et al., (2008) reported that methanol extract of the stem of Calpurnea aurea which has higher polyphenol content, also has higher antioxidant activity (3146.98 µM Fe (II)/g of dry mass) than that of the leaf extract (111.98 mM Fe (II)/g of dry mass). Ara and Nur (2009) recorded antioxidant activity of methanolic extract of Lippia alba using DPPH free radical scavenging assay to be 34.4 µg/ml. The present study also records highest antioxidant activity in correlation with the highest polyphenol content for methanolic extract. Therefore, the plant P. longifolia showing remarkably high amount of antioxidant activity could be employed in Ayurveda formulations and further research would isolate the active principle of the antioxidants.
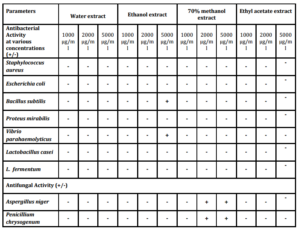
For the Bacterial susceptibility tests, Penicillin antibiotic was used as a positive control. The diameter of the inhibition zone was measured in millimetre. Most of the fractions showed signs of inhibition with inhibition zone ranging from 0 to 18 mm. The ethyl acetate and the ethanol extract showed particularly strong inhibition (Table 2). Before well diffusion test was carried out, a broth dilution test was done to see the possible antimicrobial activity of the drug.
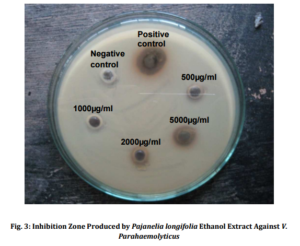
Methanolic extract showed the inhibition of microbial growth in both. The organic extract of Ethanol when used at a concentration of 5 mg/ml showed antibacterial activity against Vibrio parahaemolyticus (Fig. 3) and Bacillus subtilis representing both Gram positive and Gram negative bacterial groups as well as pathogenic and non pathogenic groups. The growth of the most of the other bacterial species like Staphylococcus aureus, Escherichia coli, Proteus mirabilis, Lactobacillus casei and L. fermentum could not be retarded by the plant extract at any concentrations tested. However, only methanol extract showed antifungal activity to both the isolates tested. Similarly, Bhat et al., (2011) reported the diethyl ether-methanol extract of the leaves of Calycopteris floribunda and its petroleum ether-butanol fraction exhibited a good antibacterial activity against Bacillus cereus, B. subtilis and Staphylococcus aureus. Adedapo et al., (2008) studying the antibacterial property of methanolic extract of Calpurnia aurea showed that a broad spectrum antibacterial activity could be noticed at 5mg/ml against various species of Staphylococcus, Micrococcus and Streptococcus. Ghalem and Mohamed (2009) studied Pistacia vera for its antimicrobial activity and showed that antimicrobial activity against Gram negative as well as Gram positive bacteria was recorded with Proteus sp. being inhibited greatest. Similarly, antibacterial activities of Turkish pollen and propolis extract were investigated against 13 different species of bacterial pathogens by Basim et al., (2005). Adeyemi et al., (2008) tested the ethanolic extracts of Alchornea cordifoila against Helicobacter pylori, Salmonella typhi, S. enterotidis and Escherichia coli for its susceptibility. They reported that all the organisms were sensitive to the drug. Similarly, there are a numerous reports showing antibacterial activity of various plant derivatives against various Gram positive and Gram negative pathogenic as well as non pathogenic bacteria and fungi. Salar and Dhall (2010) reported 40 to 60% antimicrobial activity against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Aspergillus niger and Candida albicans with Prosopis cineraria, Capparis decidua, Tinospora cordifolia, Carissa carandas and Cordia dichotoma. Antimicrobial activity of Synedrella nodiflora was tested using petroleum ether, chloroform, acetone, methanol and distilled water extracts against three bacteria- B. subtilis, E. coli, S. aureus and two fungi- Aspergillus flavus, Candida albicans. Petroleum ether extract showed maximum activity against all the microbes tested except E. coli. Candida albicans is most susceptible to all extracts except methanol (Bhogaonkar et al., 2011).
Adedapo, A.A., Jimoh, F.O., Koduru, S., Afolayan, J.A. and Masika, P.J. (2008) “Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea”, Journal of BMC Complement Alternative Medicine, 8 53.
Publisher – Google Scholar
Adedapo, A.A., Jimoh, F.O., Koduru, S., Masika, P.J. and Afolayan, J.A. (2009) “Assessment of the medicinal potentials of the methanol extracts of the leaves and stems of Buddleja saligna”, Journal of BMC Complement Alternative Medicine, 9 21.
Publisher – Google Scholar
Adeyemi, A., Omnigbehin, E., Stella, S., Oluwatosin, O. and Jumoke, S. (2008) “Antibacterial activity of extracts of Alchornea cordifolia (Schum and Thonn) Mull.- Arg., Boerhavia diffusa (L.) and Bridellia micranthal (Hoscht) Baill. used in traditional medicine in Nigeria on Helicobacter pylori and four diarrhoeagenic bacterial pathogens”, African Journal of Biotechnology, 7(20) 3761-3764.
Aiyegoro, O.A. and Okoh, A.I. (2009) “Phytochemical Screening and Polyphenolic Antioxidant Activity of Aqueous Crude Leaf Extract of Helichrysum pedunculatum”, International Journal of Molecular Science, 10(11) 4990—5001.
Publisher – Google Scholar
Amarowicz, R., Estrella, I., Hernandez, T., Duenas, M., Troszynska, A., Agniszka, K. and Pegg R.B. (2009) “Antioxidant activity of a red lentil extract and its fractions”, International Journal of Molecular Science, 10(12) 5513-5527.
Publisher – Google Scholar
Amsterdam, J.G.C., Opperhuizen, A. and Jansen, E.H.J.M. (2005) “Accumulation of phytosterols in food evaluation of the adverse effects following the intake of high dose of phytosterols”, RIVM report 340240001.
Ara, N. and Nur, H. (2009) “In vitro antioxidant activity of methanolic leaves and flower extracts of Lippia alba.” Research Journal of Medicine and Medical Science, 4(1) 107-110.
Basim, E., Basim, H. and Ozcan, M. (2005) “Antibacterial activities of Turkish pollen and propolis extracts against plant bacterial pathogens”, Journal of Food Engineering, 77 992-996.
Publisher
Bhat, P.R., Prajna, P.S., Kumar, V., Adarsh Hegde, M. and Singh L. (2011) “Antimicrobial properties of leaves of Calycopteris floribunda Lam”, Journal of Medicinal Plants Research, 5(12) 2448-2451.
Bhogaonkar, P.Y., Dagawal, M.J., and Ghorpade, D.S. (2011) “Pharmacognostic studies and antimicrobial activity of Synedrella nodiflora (L.) Gaertn.”, Bioscience Discovery 2(3) 317-321.
Bravo, L. (1998) “Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance”, Nutrition Review, 56 317-333.
Publisher – Google Scholar
Cheeke, P.R. (1998) “Saponins: Surprising benefits of desert plants”. The Linus Pauling Institute 31 21—23.
Chen, X., Sun, C.K., Han, G.Z., Peng, J.Y., Li, Y., Liu, Y.X., Lv, Y.Y., Liu, K.X., Zhou, Q. and Sun, H.J. (2009) “Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression”, World Journal of Gastroenterology, 15(15) 1829—1835.
Publisher – Google Scholar
Chung, K.T., Wong, T.Y., Wei, C.I., Huang, Y.W. and Lin, Y. (1998). “Tannins and human health: a review”, Journal of Critical Review on Food Science Nutrition, 38(6) 421-464.
Publisher – Google Scholar
Crozier, A., Burns, J., Aziz, A.A., Stewart, A.J., Rabiasz, H.S., Jenkins, G.I., Edwards, C.A. and Lean, M. (2000), “Antioxidant flavonols from fruits, vegetables and beverages: measurements and bioavailability”, Biological Research, 33 79-88.
Publisher – Google Scholar
Dickinson, A. (2002) “Benefits of Antioxidants: May help protect against cancer”, Council of Responsible Nutrition, 79 27-31.
Dizhbite, T, Telysheva, G, Jurkjane, V., Viesturs, U. (2004) “Characterization of the radical scavenging activity of lignins-natural antioxidants”, Journal of Bioresource Technology, 95(3) 309-17.
Publisher – Google Scholar
Frankel, E.N., Kanner, J., German, J.B., Parkse, E. and Kinsella, J.E. (1993) “Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine”. Journal of Lancet, 341 454-457.
Publisher – Google Scholar
García, A., Toledano, A., Andrés, M.Ã. and Labidi, J. (2010) “Study of the antioxidant capacity of Miscanthus sinensis lignins”, Process Biochemistry, 45(6) 935-940.
Publisher – Google Scholar
Gerritsen, M.E., Carley, W.W., Ranges, G.E., Shen, C.P., Phan, S.A,, Ligon, G.F. and Perry, C.A. (1995) “Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression”, American Journal of Pathology, 147 278-292.
Ghalem, B.R. and Mohamed, B. (2009) “Antimicrobial activity evaluation of the oleoresin oil of Pistacia vera”, African Journal of Pharmacy and Pharmacology, 3(3) 92-96.
Halliwell, B. (1999) “Establishing the significance and optimal intake of dietary antioxidants: The biomarker concept”,Journal of Nutrition Review, 57 104-113.
Publisher – Google Scholar
Hertog, M.G.L., Feskens, E.J.M., Hollman, P.C.H., Katan, M.B. and Kromhout, D. (1993) “Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study”, Lancet, 342 1007-1011.
Publisher – Google Scholar
Kaur, R., Meena, A.K., Singh, B, Yadav Singh, U., Sachan, A., Pal, B. and Rao, M.M. (2009) “Review on antifungal activities of Ayurvedic medicinal plants”, Drug Invention Today, 2(2) 146-148.
Kumar, A., Rajbir Kaur and Saroj Arora. (2010) “Free radical scavenging potential of some India medicinal plants”, Journal of Medicinal Plants Research, 4(9) 2034-2042.
Leong, A.C.N., Kinjo, Y., Tako, M., Iwasaki, H., Oku, H. and Tamaki, H. (2010) “ Flavonoid glycosides in the shoot system of Okinawa Taumu (Colocasia esculenta)”, Journal of Food Chemistry, 119(2) 630-635.
Publisher – Google Scholar
Mekhfi, H., ElHaouari, M., Bnouham, M., Aziz, M., Ziyya, A. and Legssyer, A. (2006) “Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation”, Phytotherapy Research, 2 135-139.
Publisher – Google Scholar
Muldoon, M.F. and Kritchevsky, S. (1996) “Flavonoids and heart disease”, British Medical Journal, 312 458-459.
Publisher – Google Scholar
Oliveira, M.M., Brugnera, D.F., Cardoso, M.G., Alves, E. and Roberta, H.P. (2010) “Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface”, Journal of Food Control, 21(4) 549-553.
Publisher – Google Scholar
Pouteau, C., Dole, P., Cathala, B., Averous, L. and Boquillon, N. (2003) “Antioxidant properties of lignin in polypropylene”, Journal of Polymer Degradation and Stability, 81 9—18.
Publisher – Google Scholar
Pullaiah, T. (2006) “Encyclopedia of World Medicinal Plants”, Vol. 3, Regency Publications, 20/36-G, New Delhi.
Ralph, J., Brunow, B., Boerjan, W. and Lignins. (2007) “Encyclopedia of Life Sciences”. doi: 10.1002/9780470015902. 1-2.
Sadasivam, S. and Manikam, A. (2008) “Biochemical Methods”, 3rd edition, New Age International Publishers, New Delhi. pp. 203 — 204.
Salar, R.K. and Dhall A. (2010) “Antimicrobial and free radical scavenging activity of extracts of some Indian medicinal plants”, Journal of Medicinal Plants Research, 4(12) 2313-2320.
Sawant, M., Isaac, J.C. and Narayanan, S. (2004) “Analgesic studies on total alkaloids and alcohol extracts of Eclipta alba (Linn.) Hassk”, Journal of Phytotherapy Research, 18(2) 111-113.
Publisher – Google Scholar
Silva, A.F., de Andrade, J.P., Machado, K.R., Rocha, A.B., Apel, M.A., Sobral, M.E., Henriques, A.T. and Zuanazzi, J.A. (2008) “Screening for cytotoxic activity of extracts and isolated alkaloids from bulbs of Hippeastrum vittatum”, Journal of Phytomedicine, 15(10) 882-885.
Publisher – Google Scholar
Sivakumar, V., Dhana Rajan, M.S. and Riyazullah. (2010) “Preliminary phytochemical sreening and evaluation of free radical scavenging activity of Tinospora cordifolia”, International Journal of Pharmacy and Pharmaceutical Science, 2(4) 186-188.
Souri, E., Amin, G., Hassan Farsam, Hassan Jalalizadeh and Saba Barezi. (2008). “Screening of thirteen medicinal plant extracts for antioxidant activity”, Iranian Journal of Pharmaceutical Research, 7 (2) 149-154.
Staerk, D., Kesting, J.R., Sairafianpour, S., Witt, M., Asili, J., Emami, S.A. and Jaroszewski, J.W. (2009) “Accelerated dereplication of crude extracts using HPLC—PDA—MS—SPE NMR: Quinolinone alkaloids of Haplophyllum acutifolium.”, Journal of Phytochemistry, 70(8) 1055-1061.
Publisher – Google Scholar
Tohidpour, A., Sattari, M., Omidbaigi, R., Yadegar, A. and Nazemi, J. (2010) “Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA)”, Journal of Phytomedicine, 17(2) 142-145.
Publisher – Google Scholar
Tung, N.H., Song, G.Y., Nhiem, N.X., Ding, Y, Tai, B.H., Jin, L.G., Lim, C.M., Hyun, J.W., Park, C.J., Kang, H.K. and Kim, Y.H. (2010), “Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity”, Journal of Agriculture Food Chemistry, 58(2) 868-874.
Publisher
Turner, T. (2006) “Antioxidant Vitamins Against Cancer”. Retrieved from, http://ezinearticles.com/Antioxidant-Vitamins-Against-Cancer/html.
Urquiaga, I. and Leighton, F. (2000) “Plant Polyphenol Antioxidants and Oxidative Stress”, Journal of Biological Research, 33(2).
Weng, A., Bachran, C., Fuchs, H., Krause, E., Stephanowitz, H. and Melzig M.F. (2009) “Enhancement of saporin cytotoxicity by Gypsophila saponins–more than stimulation of endocytosis”, Journal of Chemical Bioliolgy Interaction 30(3) 424-429.
Publisher – Google Scholar
Yang, C.S., Landau, J.M., Huang, M.T. and Newmark, H.L. (2001) “Inhibition of carcinogenesis by dietary polyphenolic compounds”, Journal of Annals Review on Nutrition, 21 381-406.
Publisher – Google Scholar
Yokosuka, A., Sano, T., Hashimoto, K., Sakagami, H. and Mimaki, Y. (2009) “Triterpene glycosides from the whole plant of Anemone hupehensis var. japonica and their cytotoxic activity”, Journal of Chemistry and Pharmaceutical Bulletin (Tokyo), 57(12) 1425-1430.
Publisher – Google Scholar
Zargar, M., Azizah, A.H., Roheeyati, A.M., Fatimah, A.B., Jahanshiri, F. and Pak-Dek, M.S. (2011) “Bioactive compounds and antioxidant activity of different extracts from Vitex negundo leaf”, Journal of Medicinal Plants Research, 5(12) 2525-2532.
Zhang, J., Yu, Y., Liu, D. and Liu, Z. (2007) “Extraction and composition of three naturally occurring anti-cancer alkaloids in Camptotheca acuminata seed and leaf extracts”, Journal of Phytomedicine, 7(1) 50-56.
Publisher – Google Scholar