Introduction
The use of x-rays in modern medicine has revolutionised the accuracy of diagnostics. However with over 46 million now taken annually in the UK, medical x-rays are the largest single artificial source of radiation exposure for the UK population. This amounts to a radiation dose of 0.4mSv per individual, of a total of 2.7mSv received annually from all sources of ionising radiation1. For those exposed to repeated x-rays, this figure will be much higher.
X-ray use in Orthopaedic trauma with portable image intensifiers has been established for quite some time. This has enabled the expansion of the number of surgical trauma techniques as well as significantly improving the quality and speed of surgical interventions, such as intramedullary nailing of long bones.
The value and importance of image intensifiers is not to be understated, and the risk from radiation to individual patients following such procedures is minimal2. Although radiation exposure has declined with technological advances and improved safety practices, surgeons and operating theatre staff working with image intensifiers regularly are exposed to higher levels of radiation, and the long term effects of this occupational exposure are less clear.
Local guidelines for the safe use of image intensifiers in theatre, minimising radiation exposure to staff, are in place. The aim of this study was to assess compliance to these guidelines in orthopaedic theatres at a North Western trauma centre.
Materials and Methods
Procedures requiring the use of an image intensifier in the trauma theatre were prospectively audited during June 2012. 20 non-consecutive procedures were observed. The inclusion criteria were: any orthopaedic procedure requiring the use of image intensifier. Theatre staff, operating surgeon, surgeon’s assistant, and radiographer were blinded, and not aware neither of the audit taking place nor the guidelines being assessed.
The local image intensifier radiation safety guidelines were used to define the standards of the audit.
Wearing of Personal Protective Equipment
All staff should be wearing provided protection correctly. Lead rubber gowns should be worn, fastened at the sides to provide protection around the body and of sufficient length to cover the femora.
Protective clothing must be stored correctly to prevent damage. Lead rubber gowns must be hung and not folded to preserve the integrity of the lead. These should be screened annually for defects.
All staff should follow instructions of the radiographer.
Radiation Warning Signs
Radiation warning signs should be displayed outside all entrance doors to theatre. These should be switched on whilst the image intensifier is in use.
Only essential staff should remain in the controlled area whilst the image intensifier is switched on.
Minimising Exposure
No one should be in the direction of the primary beam during radiography. This was assessed by the presence or absence of the surgeon’s hand in the images acquired.
Exposure and screening times should be kept to a minimum. Pulsed radiography reduces the effective dose by 70%, and should be used during screening, unless determined by the radiographer.
The image intensifier should be in the upper position when used, where practicable. The positioning of the C-arm is shown in figure 1.
Figure 1: The C-arm Should Be Positioned with the Image Intensifier in the Upper Position, and the Radiation Source of the X-Ray Beam in the Lower Position
For each procedure, the dose-area product (DAP) and screening time were also obtained. The DAP (Gy/cm2) was converted to effective dose (mSv) by multiplying DAP by 0.183.3
DAP is a measure of the absorbed dose (in Grays) multiplied by the area irradiated, giving a value in Grays per cm2. The effective dose is a measure of the effect of the absorbed dose of radiation on tissues, giving a value in Sieverts (Sv). The International Committee for Radiation Protection has given a risk estimate of 4% per Sv for radiation induced cancer in adults4.
Results
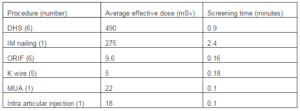
Nine different procedures were observed; the average effective dose and screening times are shown in table 1 for each procedure type. The average effective dose across all procedures was 0.22366 mSv (highest 1.03944; lowest 0.00183). The highest effective doses were in dynamic hip screws (DHS) and intramedullary (IM) nailing procedures. The average screening time was 0.54 minutes (longest 2.4 minutes; shortest 0.1 minutes). The other procedures observed were: open reduction and internal fixation (ORIF) of patella, ankle (2), wrist (2) and elbow; K wire of hand (5); manipulation under anaesthesia of shoulder and intra-articular injection of ankle. All of these were associated with a lower effective dose and screening time.
Table 1: Average Effective Dose and Average Screening Time for Each Type of Procedure Observed
Wearing of Personal Protective Equipment
Lead gowns were provided outside theatre, but there was no provision of thyroid shields for general usage. In all procedures observed, members of staff present in theatre were either not wearing lead gowns or were wearing them incorrectly. In 50% of cases at least one member of staff in the controlled area was not wearing a lead rubber gown. Lead gowns should be fastened to provide 360° protection around the body, and cover the femora to provide adequate protection to haematopoietic marrow in the femora. Incorrect wearing included unfastened gowns or gowns that were too short (worn above the knee). Figure 2 shows the occupation of those not wearing gowns, or wearing them incorrectly. This was not specific to one particular role; as figure 2 demonstrates, this includes all members of the theatre team. When radiographers issued instructions regarding the wearing of lead gowns, these were not followed in several cases.
Of particular concern was the observation that during none of the 20 audited procedures did any of the theatre staff wear a thyroid shield. Whilst wearing of thyroid shields is not mentioned specifically in the local guidelines, this is notable given the established link between ionising radiation and incidence of thyroid carcinoma6. Possible causes for non-compliance with this important protective measure are lack of provision of thyroid shields and the generally accepted discomfort of wear. Lack of awareness about the correct usage of protective equipment, or complacency amongst staff are other possibilities. It has been demonstrated that doctors of all grades are unaware of the amount of radiation exposure from common radiological procedures, with 97% underestimating the actual dose of radiation involved5.
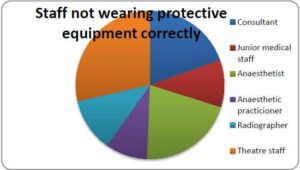
Figure 2: Occupation of Staff Members Either Not Wearing Lead Rubber Gowns, or Wearing Gowns Incorrectly
In 19 procedures, lead gowns were not stored correctly. Lead rubber gowns should be hung without folding when not in use to maintain the integrity of the lead.
In 4 cases, instructions given by the radiographer were not followed. These all related to the wearing of protective equipment.
Radiation Warning Signs
Radiation warning signs are in place outside all entrances to theatre, in the form of illuminated boxes above theatre doors (figure 3) with additional signs advising staff of the use of X-rays and not to enter without permission. However, the warning signs were not switched on during any procedure observed. In eight cases a member of staff entered theatre whilst the image intensifier was in use; this may have been reduced if warning signs had been displayed. This task could be appointed to a member of theatre staff or the radiographer, prior to use of the image intensifier, for orthopaedic theatres.

Figure 3: Illuminated Signs Displayed outside the Entrance to Theatre when X-Ray is in Use
Minimising Exposure
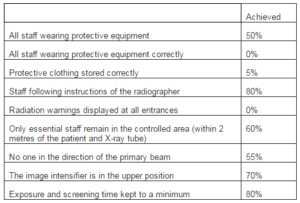
To minimise exposure, individuals must stand as far away from the radiation source as is practicable, because the energy emitted varies as the inverse square of the distance from the source. In nine of the cases, at least one part of the surgeon’s limb was in the direct line of the beam, resulting in this being visible in the image produced. The image intensifier was used in the lower position in six cases, with only one of these being at the request of the surgeon (due to difficulty in obtaining the required views when positioning the C-arm with the image intensifier in the upper position). Using pulsed radiography reduces the effective dose by 70%; however, this was not used in four cases at the discretion of the radiographer. Pulsed radiography cannot be used where continuous screening is required; however, continuous screening was not used in any cases observed. Table 2 summarises the results observed.
For certain procedures a mini C-arm, controlled by the surgeon, can be used in place of a standard C-arm. These are not suitable for all procedures, and require appropriate staff training. However, as a radiographer is not required to be present in theatre, the number of staff exposed is reduced. Several studies have shown the effective dose received by the surgeon to be lower when using the mini C-arm when compared to that received using the standard C-arm7-9.
Table 2: Summary of Results Showing Percentage Compliance to Local Image Intensifier Radiation Safety Guidelines
Discussion
Exposure to ionising radiation increases the future risk of malignancies; the mechanism is well understood, but quantifying the level of risk less so. The incidence of malignancy increases in a linear dose-response fashion. The effects of radiation are most significant in rapidly dividing tissues, including: – thyroid, blood, epithelium and bone. For solid tumours, the time interval between radiation exposure and malignancy is around 10-15 years; this is much shorter for radiation-induced leukaemias10. Any radiation dose carries an associated risk of cancer induction; there is no level that can be guaranteed as ‘safe’. The annual exposure limit for staff is 20mSv, equivalent to nine years of average background radiation dose in the UK, or two computerised tomography (CT) abdomen and pelvis. This equates to an additional lifetime risk of 1 in 1000 of fatal cancer11.
Quantifying the dose received by staff in theatre is difficult, as they do not receive the full dose as measured for the patient. However, the radiation dose to staff is determined by the dose to the patient, the duration, distance from the source and degree of shielding12. The highest dose is received by tissues in the direct beam from the X-ray source; in almost half of cases observed, part of the surgeon’s hand was in the image. Unless in the direct beam, theatre staff are exposed to scattered radiation. The scattered radiation dose is more difficult to estimate, but nevertheless leads to long term exposure to low dose radiation. Numerous papers have explored the risk of long term low dose radiation exposure and the lifetime risk of developing cancer in orthopaedic surgeons. However, there is no one paper that has been able to quantify this risk. An accumulated dose per procedure of 65µSv over long-term exposure has been reported to increase the risk of thyroid cancer in surgeons13.
The levels of exposure vary considerably between different types of procedure; however, this level is often exceeded in procedures requiring large numbers of images, such as DHS and IM nailing13. The average effective dose in this study was 0.22mSv, equivalent to one hip x-ray. In all DHS and IM procedures observed, the effective dose exceeded 65µSv (associated with increased risk of thyroid carcinoma over long term exposure). This was the dose received by the patient; that received by the surgeon cannot be accurately assessed without the use of a dosimeter. However, previous studies using dosimeters attached in front of the thyroid gland, have shown the dose received by the surgeon to regularly exceed this figure during such procedures14.
The beam intensity decreases as the square of the distance from the tube; therefore it is recommended that staff not wearing protective equipment be positioned at least 2 metres from the source. In theatres this may not be practicable, therefore lead lined protective barriers should be used to minimise exposure. The IAEA recommends wearing of lead gowns of at least 0.35mm lead equivalence when the X-ray source operates at above 100kV, which mobile C-arm equipment does for certain procedures such as lumbar spine imaging, and 0.25mm when the X-ray source operates at below 100kV. At 100kV, radiation transmission for 0.25mm equivalent lead gowns is approximately 17%; this decreases to 5% for 0.5mm equivalent lead gowns15. The IAEA also recommends wearing of protective eyewear and head/face protection for orthopaedic surgeons.
The lens of the eye is one of the most radiosensitive tissues in the body16; whilst there is currently no evidence of increased incidence of cataracts in orthopaedic surgeons, this has been demonstrated for interventional cardiologists due to occupational radiation exposure17. As the number and complexity of orthopaedic procedures involving the use of image intensifiers increases, this may be a future cause for concern.
When lead gowns are worn, the highest level of exposure is to the head and neck of the surgeon18. Long term exposure to radiation increases the risk of thyroid carcinoma; 85% of papillary carcinomas are radiation-induced6. The use of a thyroid shield decreases the effective dose to the thyroid by up to 70 times that received without19, 20, and may reduce the total effective dose received by more than half21.
This study has demonstrated poor compliance with thyroid shield wear by orthopaedic surgeons and theatre staff. The vulnerability of the thyroid gland to radiation induced neoplastic transformation has been demonstrated. Given previous research suggesting a potentially hazardous dose per procedure to the thyroid of 65µSv is regularly exceeded during standard orthopaedic operations, we think it prudent that staff working in the radiation scatter zone should be encouraged to wear thyroid shields.
References
1.Hart, D., Hillier, M., Shrimpton, P. (2012). “HPA-CRCE-034 – Doses to Patients from Radiographic and Fluoroscopic X-ray Imaging Procedures in the UK — 2010 Review,” 2012 Jun.
Publisher
2. Malek, S., Davies, E., Malek, I. A., Rawal, A., Singh, A., Harvey, R. A. (2007). “Trauma Surgery and Risk of Radiation Injury to Patients,” European Journal of Orthopaedic Surgery and Traumatology. 2007; 17(1):23—8.
Publisher – Google Scholar
3. Stratis, A. I., Anthopoulos, P. L., Gavaliatsis, I. P., Ifantis, G. P., Salahas, A. I., Antonellis, I. P. et al. (2009). “Patient Dose in Cardiac Radiology,” Hellenic Journal of Cardiology. 2009; 50(1):17—25.
Publisher – Google Scholar
4. The 2007 Recommendations of the International Commission on Radiological Protection. (2007). ICRP Publication 103, Annals of the ICRP, 2007; 37(2-4):1—332.
Publisher
5. Shiralkar, S., Rennie, A., Snow, M., Galland, R. B., Lewis, M. H., Gower-Thomas K. (2003). “Doctors’ Knowledge of Radiation Exposure: Questionnaire Study,” British Medical Journal. 2003; 327(7411):371—2.
Publisher – Google Scholar
6. Greenspan, F. S. (1977). “Radiation Exposure and Thyroid Cancer,” Journal of the American Medical Association. 1977; 237(19):2089—91.
Publisher – Google Scholar
7. Giordano, B. D., Baumhauer, J. F., Morgan, T. L., Rechtine, G. R. II. (2009). “Cervical Spine Imaging Using Mini-C-Arm Fluoroscopy: Patient and Surgeon Exposure to Direct and Scatter Radiation,” Journal of Spinal Disorders and Techniques. 2009; 22(6):399—403.
Publisher – Google Scholar
8. Giordano, B. D., Baumhauer, J. F., Morgan, T. L., Rechtine , G. R. II. (2009). “Patient and Surgeon Radiation Exposure: Comparison of Standard and Mini-C-Arm Fluoroscopy,” Journal of Bone and Joint Surgery – Series A. 2009; 91(2):297—304.
Publisher – Google Scholar
9. Noordeen, M. H. H., Shergill, N., Twyman, R. S., Cobb, J. P., Briggs, T. (1993). “Hazard of Ionizing Radiation to Trauma Surgeons: Reducing the Risk,” Injury. 1993; 24(8):562—4.
Publisher – Google Scholar
10. “Radiation and your Patient: A Guide for Medical Practitioners,” (2001). Annals of the ICRP, 2001; 31(4):5—31.
Publisher
11. “Dose Comparisons for Ionising Radiation,” [Internet]. Health Protection Agency; [cited 2012 Sep. 26]. Available from:http://www.hpa.org.uk/Topics/Radiation/UnderstandingRadiation/UnderstandingRadiationTopics/DoseComparisonsForIonisingRadiation/
Publisher
12. Mehlman, C. T., DiPasquale, T. G. (1997). “Radiation Exposure to the Orthopaedic Surgical Team during Fluoroscopy: “How Far away is Far Enough?”,” Journal of Orthopaedic Trauma. 1997; 11(6):392—8.
Publisher – Google Scholar
13. Fuchs, M., Schmid, A., Eiteljörge, T., Modler, M., Stürmer, K. M. (1998). “Exposure of the Surgeon to Radiation during Surgery,” International Orthopaedics. 1998; 22(3):153—6.
Publisher – Google Scholar
14. Devalia, K. L., Peter, V. K., Madanur, M. A., Braithwaite, I. J. (2006). “Exposure of the Thyroid to Radiation during Routine Orthopaedic Procedures,” Acta Orthopaedica Belgica. 2006; 72(5):615—20.
Publisher – Google Scholar
15. Christodoulou, E. G., Goodsitt, M. M., Larson, S. C., Darner, K. L., Satti, J., Chan, H. P. (2003). “Evaluation of the Transmitted Exposure through Lead Equivalent Aprons Used in a Radiology Department, Including the Contribution from Backscatter,” Medical Physics. 2003; 30(6):1033—8.
Publisher – Google Scholar
16. Ainsbury, E. A., Bouffler, S. D., Dörr, W., Graw, J., Muirhead, C. R., Edwards, A. A. et al. (2009). “Radiation Cataractogenesis: A Review of Recent Studies,” Radiation Research. 2009; 172(1):1—9.
Publisher – Google Scholar
17. Vano, E., Kleiman, N. J., Duran, A., Rehani, M. M., Echeverri, D., Cabrera, M. (2010). “Radiation Cataract Risk in Interventional Cardiology Personnel,” Radiation Research. 2010; 174(4):490—5.
Publisher – Google Scholar
18. Miller, M. E., Davis, M. L., MacClean, C. R. et al. (1983). “Radiation Exposure and Associated Risks to Operating Room Personnel during Use of Fluoroscopic Guidance for Selected Orthopaedic Surgical Procedures,” Journal of Bone and Joint Surgery – Series A. 1983; 65(1):1—4.
Publisher – Google Scholar
19. Müller, L. P., Suffner, J., Wenda, K., Mohr, W., Rommens, P. M. (1998). “Radiation Exposure to the Hands and the Thyroid of the Surgeon during Intramedullary Nailing,” Injury. 1998; 29(6):461—8.
Publisher – Google Scholar
20. Tse, V., Lising, J., Khadra, M., Chiam, Q., Nugent, R., Yeaman, L. et al. (1999). “Radiation Exposure during Fluoroscopy: Should We Be Protecting Our Thyroids?,” Australian and New Zealand Journal of Surgery. 1999; 69(12):847—8.
Publisher – Google Scholar
21. Theocharopoulos, N., Perisinakis, K., Damilakis, J., Papadokostakis, G., Hadjipavlou, A., Gourtsoyiannis, N. (2003). “Occupational Exposure from Common Fluoroscopic Projections Used in Orthopaedic Surgery,” Journal of Bone and Joint Surgery – Series A. 2003; 85(9):1698—703.
Publisher – Google Scholar







