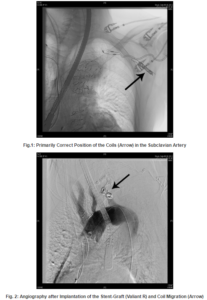
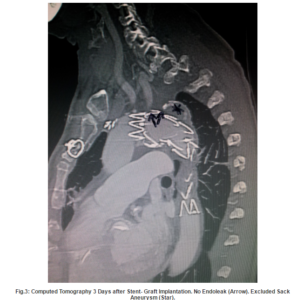
Discussion
Endovascular occlusion of the subclavian artery has been reported to be successfully performed in cases of intentional left subclavian artery coverage directly by the endograft (Peterson 2008, Meyer 2009). Furthermore, retrograde aneurysm sack perfusion by reverse flow of the SA is also reported.
In many centres, therefore a preventive occlusion procedure is performed simultaneous or prior to the EVAR procedure. Various techniques can be applied for this task such as coiling or placing an endovascular plague. Nevertheless, this procedure- even if it looks rather simple for the interventionalist- can be affected seriously , as described in our case, when the pressure and flow situation in the aortic arch and the subclavian artery are changing significantly.
In retrospect, it would have been smarter placing the stent graft first and controlling the effectiveness of the exclusion of the aneurysm sack. Then, if a retrograde perfusion of the SA would have been detectable, the coils could have been placed. With this approach a centrifugal flowin the SA definitively would have been avoided. The common technical procedure: first stent-graft and then deployment of coils was not performed in this specific case, due to the huge lumen diameter and the broad basis of the SA at her origin that might bear the risk of producing an endoleak. Moreover, the complication might not have happened if bigger coils had been used.
Our case report might be a helpful contribution in decision making towards emergent and hopefully successful intervention in the onset of this complication.
Comment
Coil migration may leads to serious consequences in endovascular aortic surgery, in particular to embolization and consequent ischemia in the area of peripheral vessels. In the present case, the device (custom made goose- snare) provides a secure technique for retrieval of the dislocated coils.
References
Garzon, G., Fernandez-Velilla, M., Marti, M., Acitores, I., Ybanez, F. & Riera, L. (2005). “Endovascular Stent-Graft Treatment of Thoracic Aortic Disease,” Radiographics 25:229-224
Publisher – Google Scholar
Gottardi, R., Funovics, M., Eggers, N., Hirner, A., Dorfmeister, M., Holfeld, J., Zimpfer, D., Schoder, M., Donas, K., Weigang, E., Lammer, J., Grimm, M. & Czerny, M. (2008). “Supra-aortic Transposition for Combined Vascular and Endovascular Repair of Aortic Arch Pathology,” The Annals of Thoracic Surgery 86:1524-1529
Publisher – Google Scholar
Meyer, C., Probst, C., Stunk, H., Schiller, W. & Wilhelm, K. (2009). “Second- Generation Amplatzer Vascular Plug (AVP) for the Treatment of Subsequent Subclavian Backflow Type II Endoleak after TEVAR,” CardioVascular and Interventional Radiology 32: 2164-2167
Publisher – Google Scholar
Peterson, M. D., Wheatley, G. H., Kpodonu, J., Williams, J. P., Ramaiah, V. G., Rodriguez-Lopez, J. A. & Dietrich, E. B. (2008). “Treatment of Type II Endoleaks Associated with Left Subclavian Artery Coverage during Thoracic Aortic Stent Grafting,” The Journal of Thoracic and Cardiovascular Surgery 136: 1193-1199
Publisher – Google Scholar
Younes, H. K., Davies, M. G., Bismuth, J., Naoum, J. J., Peden, E. K., Reardon, M. J. & Lumsden, A. D. (2010). “Hybrid Thoracic Endovascular Aortic Repair: Pushing the Envelope,” Journal of Vascular Surgery 51:259-266
Publisher – Google Scholar