Introduction
The history of brain banking goes back as far as a century ago. Established collections of human postmortem brain tissues stored in such brain banks have, over the years, boosted research toward many significant discoveries, enhancing our understanding of central nervous system function(s). More significantly, these brain banks still continue to support current research on neurodegenerative disorders (Ravid and Grinberg 2008; Beaulieu 2004; Bell and Ironside 1997). Many of these neurodegenerative diseases occur exclusively in humans and for that reason their pathogenesis can only be investigated in the human brain tissue. Consequently, accessibility to carefully screened postmortem human brain tissues is crucial for unveiling the mysteries of neurodegeneration (Cambon-Thomsen 2004). In order to achieve such a goal, any human brain donated for research must be carefully screened and categorized based on clinical and pathological data (Beaulieu 2004; Cassel 1998). Concurrently, the best diagnostic classification possible must be pursued through maximal scientific utility of samples in clinical investigations. Therefore, employing reliably established and consistent methodologies in investigations involving brain tissues is the most important and fundamental tenet of success for research in neurosciences (Cruz-Sánchez et al. 1997). Based on the aforementioned grounds, increasing demand for brain tissue combined with rising research activities in neurological diseases necessitate the establishment of a universally and functionally reliable brain and tissue bank in Greece.
Organizational, Operational and Safety Guidelines for Substantive Brain Banking Function
Key to a successful function of a reliable brain and tissue bank is the establishment of accredited procedures pertaining to the influx, testing, deposition and ensuing dispensation of material(s) for research and training in neurodegenerative diseases. To this end, the following organizational and operational procedures will be established in the intended brain and tissue bank in Greece (Figure 1): 1. Brain and tissue donation to the bank will be activated through established calls, announcements and relevant publicity conforming to currently held and internationally accepted standards. Eligibility criteria pertaining to potential donors will be adhered to and applied according to such standards. 2. Incoming samples will be diagnostically screened for identification purposes and classified for further processing into entry records. 3. Samples will be prepared for further deposition and deposited for preservation in accordance with strict safety and potential biohazard standards currently if effect worldwide. 4. Samples and tissue(s) will be dispensed (in parts or as a whole) to the research community for further investigation upon request. Procedures entailing pertinent requests, petitions and applications on behalf of interested researchers (or research groups and organizations), approval by the bank, and dispensation of requested material(s) will be in place to ensure adherence to all scientific and ethical guidelines in effect worldwide. All relevant information will be electronically available to the entire scientific community as part of the incorporation of the Greek brain bank into the worldwide network of brain banks. Key aspects of the aforementioned organization and function of the brain and tissue bank are dwelled on below (vide infra).
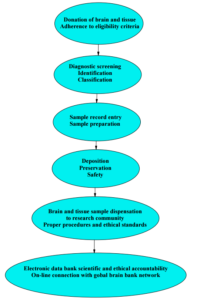
Fig. 1: Procedures and Guidelines Pertaining to Brain and Tissue Sample Bank Organizational and Operational Functions
One of the most important issues of brain banking is safety of the personnel involved in the acquisition and handling of patient samples. The importance and implementation of personnel safety rests heavily on full appreciation of the risks involved (Ferrer et al. 2008). For that reason, protective clothing should be worn during all activities before and after autopsy, with attention paid to the protection of hands, eyes, mouth and nose (Gindro and Mordini 1998). Based on these facts, the main risks in brain banking emerge during autopsy and procedures following autopsy, including preparation of cryostat sections and handling of fixed prion disease tissue. Under these circumstances, the medical staff involved in brain banking should be fully trained and justifiably competent. Hence, following proper training they should read, understand and sign all protocols describing the steps involved in brain specimen tissue collection (Kearney 1998). Furthermore, technicians should a) wear special bio-safety apparel, such as gloves, gowns, face masks, and hair and foot covers, in order to minimize contamination, and b) comply with the strict biosafety guidelines in effect (Hakimian and Korn 2004; Vonsattel et al. 1995; Von Versen 1999). In addition, the medical staff should be fully immunized against hepatitis A and B, and protected against certain pathogens leading to tuberculosis or malaria. They should also be aware of the fact that the process of brain tissue collection involves three steps: a) the autopsy procedure, b) the stage immediately following autopsy, and c) the post-fixation stage. An essential safety issue in the post-fixation stage is that containment level 2 and/or 3 facilities, depending on the nature of involved biohazards required for virulent pathogens, such as those in Creutzfeldt-Jakob disease (CJD). Collectively, the main threat to human health from such infective agents is accidental inoculation of infected material through needle-stick and cutting injuries (Katelaris et al. 1994).
The North Greek brain bank will also establish a Tissue Procurement Facility (TPF), which will collect and provide needed tissue specimens for neuroscientists to support brain-related research. TPF activities include collection and preservation of a) fresh and frozen normal tissue(s) from excess surgery and autopsy material, b) serum specimens from patients, and c) maintenance of a tissue database with links to clinicopathological data, collectively providing the basis for comparative investigations (Ahman et al. 2008; Suh et al. 2009).
In line with the above bank operations, in the beginning of an autopsy process, body weight, height and skull size are measured. Next, cerebrospinal fluid (CSF) is extracted in situ from the lateral ventricles by transcallosal puncture and a blood sample is retrieved from the aorta. The brain is measured in both weight and volume, and is digitally photographed using an HD camera. Most often, brain samples are fixed in 4% paraformaldehyde for 3 to 5 weeks (Dodd et al. 1988; Ferrer et al. 2007).
Frozen tissue samples should preferably be stored at -80 oC or below that temperature, although for certain types of tissue storage at -20 oC may be adequate. In order to examine gene expression in brain tissue samples, RNA should be extracted with an RNA Stabilization Solution, which is an aqueous tissue storage reagent that rapidly permeates most tissues to stabilize and protect RNA in fresh brain specimens. It eliminates the need to immediately process or freeze samples; the specimen can simply be submerged in RNAlater Solution and stored for analysis at a later date. Samples in RNAlater Solution can be stored for extended periods of time, under conditions where RNA degradation would normally take place rapidly. Tissues are stored indefinitely in RNAlater Solution at -20°C or lower. Storage at —20°C can also be used for archival samples. Therefore, there is a reason for having intermediate and deep freeze facilities for storing brain tissue samples. To this end, storage facilities will be configured so as to a) store brain tissues according to origin, state of pathology, and purpose of investigation, and b) use brain tissues in specific research tasks in short and long term investigations.
Furthermore, -80 oC freezers should have proper back-up and alarm systems in case of failure, and access should be limited to trained technical staff available on a 24 hour basis. Fresh frozen tissue is optimal for genomics and proteomics research studies, while formalin-fixed tissue is suitable for neuropathological and histological analysis. Specific anatomical regions as well as multiple sclerosis (MS) areas with lesions are extracted for further processing (Duyckaerts et al. 1993; Hasson and Schneiderman 1995). Despite the aforementioned advantages, use of frozen tissue samples has some drawbacks. Specifically, problems arise because a) facilities for snap-freezing of fresh specimens are not always available in all clinics, b) transportation of frozen tissue may be difficult, especially between hospitals, and use of special holding fixatives may lead to loss of antigen reactivity, and c) long-term storage of frozen tissue also leads to loss of antigen reactivity (Daneshtalab et al. 2009). Consequently, shorter postmortem periods are needed and appropriate freezing procedures should be adopted to preserve tissue quality (Perry and Perry 1983). Such a process can be used in conjunction with immunohistochemical and histological staining methods, whereby brain tissue is fixed in formalin and paraffin (Vonsattel et al. 1995). Collectively, storage facilities in the brain bank will be configured so as to a) store brain tissues according to origin, state of pathology, and purpose of investigation, and b) use brain tissues for specific research tasks in short and long term investigations.
The World Brain Bank Network
It’s almost impossible to list all current active brain banks around the world. The globally established network of brain banks, however, indicates that there may be more than one hundred such banks worldwide. More specifically, “BrainNet Europe” exists in Europe and has been funded by the European Commission through “Life Sciences” of the 6thFramework Program (Alafuzoff et al. 2006; Cruz-Sánchez et al. 1995). It consists of nineteen established brain banks across Europe and is coordinated by the Centre for Neuropathology and Prion Research Ludwig-Maximilians-University of Munich, in Germany. On the other hand, there are currently forty six brain banks in the United States, including many Alzheimer’s Disease Centers. Moreover, there are several brain bank centers in Australia, China, Japan, India, and New Zealand (Bidaut-Russell et al. 1995). This vast brain bank network provides access of researchers and neuroscientists to a variety of brain and CNS tissue samples. As a result, they support research leading to significant developments toward understanding the genetic basis of neurodegenerative disorders (Beaulieu 2004). Tissue samples in those banks derive from postmortem or surgical procedures. To strengthen its quintessential role in research, “BrainNet Europe” has also developed a central database incorporating a very extensive resource of brain material kept in individual banks.
In order to help researchers locate samples needed for research, common smart internet access database applications have been devised. For that reason, authorized procedures providing access to such applications should be managed in a way that meets the strictest criteria of legal and ethical conduct (Hakimian and Korn 2004). In this regard, brain banks must be mutually supportive toward sharing protocols and methodological procedures, adopting sufficiently common approaches in brain tissue handling. Through a common platform of approved of processes, they should provide individual researchers access to samples from several centers, being fully confident that the provided samples are comparable in terms of brain tissue value and quality (Jellinger et al. 1993; Kretzschmar 2009). Management of brain and tissue banking efforts in Greece a) aspires to the above standards, and b) reflects the will of the local scientific community to establish closer collaboration with the world brain bank, thereby strengthening the collective potential and resolve to seek and solve problems in neurodegeneration.
Dementia Types — Link to Brain Tissue Management
As a term, dementia describes the symptoms of a large group of illnesses including Alzheimer’s disease (AD), Parkinson’s disease, vascular dementia, Lewy Body dementia, Huntington’s disease, alcohol-related dementia, AIDS-related dementia, and Creutzfeldt-Jakob disease (Boller et al. 1980; Morris 1993; Stuss and Levine 1996). Depending on the causes, dementias are classified in four different groups: a) Fixed cognitive impairments, which include alcohol dementia, Wernicke’s encephalopathy, and Korsakoff’s psychosis, brought on by brain injury events. b) Slowly progressive dementia, such as Alzheimer’s disease, vascular dementia or Lewy body dementia (Holmes et al. 1999), which begins gradually and worsens progressively over several years. c) Rapidly progressive dementia such as Creutzfeldt-Jakob disease, a dementia that worsens over weeks to months, and d) Dementia emerging as or being a phenotypic manifestation of other diseases or a minor feature thereof, such as Cognitive Impairment in Parkinson’s disease (Hanyu et al. 2012; Knopman et al. 2003; Mufson et al. 2012).
Depending on which part of the brain is affected as a result of dementia, dementias may be classified as either cortical or subcortical (Lopez et al. 1999). Cortical dementia refers to dementia, in which brain damage primarily affects the brain’s cortex or outer layer. Cortical dementias tend to cause problems with memory, language, thinking, and social behavior (Mendez et al. 2002). Subcortical dementia affects parts of the brain below the cortex. This specific type of dementia tends to cause changes in emotions and movement in addition to problems with memory in most types of dementia, degeneration occurs in the cerebral cortex, with the pathological hallmarks of plaques and tangles depicting the emergence and onset of Alzheimer’s disease (Du et al. 2002). Nearly in all dementias, damage occurs in the cerebral cortex of the brain. This brain compartment accounts for approximately two-thirds of the brain mass and controls a) sensory functions, such as hearing, vision and touch, and b) cognitive functions such as thought, perception and communication.
The neurodegenerative dementias are clinically cha-racterized by cognitive and functional decline, ensuing from gradual loss of nervous cells in particular topographic locations and neural systems (Esiri et al. 1997; Verghese et al. 2002). As dementia progresses, the symptoms are more often, followed by gradually worsening course with heterogeneous clinical, molecular and pathological features (Bathgate et al. 2001; Neary et al. 2000).
Given the severity of neurodegeneration onset and progression, access to brain and tissue from such patients and ensuing research on the pathologoanatomical and biochemical status of the brain rises as a necessity. To this end, establishment of a reliable brain and tissue bank in North Greece is expected to provide readily accessible material for research, based on proper diagnostic procedures and tools for the evaluation of the brain, offering deep scientific insight into the molecular pathologies associated with the disease itself. Such diagnostics entail the use of a plethora of biochemical and non-biochemical techniques outlined below.
Diagnosis Tools – Brain Proteomics
One of the most important advances in today’s neuroscience involves development of brain proteomics, the study of the proteome, i.e. all of the proteins expressed in the brain (Bayés et Al. 2009; Celis et al. 1998; Fountoulakis 2001). In the recent past, significant strides have been made toward understanding specific mechanisms involved in Alzheimer’s disease neurodegeneration (Cacabelos et al, 2012; Korolainen et al. 2010). These mechanisms are engaged in an intricate interplay between genetic and environmental factors, which include oxidative stress, inflammation, mitochondrial dysfunction, excitotoxicity and iron deposition (Dudley et al. 2011). Recently, a number of challenging proteomic studies has been carried out in the field of Alzheimer’s research (Perry and Perry 1983; Stoop et al. 2010; Yuan and Desiderio 2005). This research encompasses proteomic studies in humans, where tissue samples are recovered from patients with neurodegenerative disorders (Micheva et al. 2012). These samples (solid—liquid) are extracted either directly from the brain during autopsy or from CSF (Reiber 1994; Xu et al. 2006; Zhang et al. 2005). On the other hand, proteomic studies on animal models are more easily implemented and involve various strains of mice and rats, and non-human primates (Yao et al. 2009). In addition, genetically modified animals, which carry “suspect” genes involved in the pathogenesis of neurodegenerative diseases, have helped the scientific community considerably to understand many of the pathways involved in the progression of the disease (Gillardon et al. 2007; Hauptmann et al. 2009; Oddo et al. 2003). Undoubtedly, gaining knowledge in the field has been aided by brain tissue protein profiling, a modern approach used in proteomic studies. It entails direct neuropathological examination of brain tissue samples (Sposny and Hewitt 2012). To this end, proteomic analysis of various brain regions has revealed a disparity in gene expression, which includes alternative splicing, post translational modifications and methylation (Ward et al. 2009). Considerable help in this regard has come from basic methods in brain proteomics, including two-dimensional gel electrophoresis (2D-PAGE), sodium dodecyl sulfate (SDS) PAGE analysis as well as multi-dimensional liquid chromatography (LC) followed by mass spectrometry (MS) (Kiehntopf et al. 2007; O’Farrell 1975; Tumani et al. 2012; Weiss et al. 2009). Through such methods, proteomic techniques have been able to investigate and pinpoint differences in protein expression between normal and diseased tissue. The same techniques are also used to determine the structure and function(s) of proteins after post-translational modifications, such as glycosylation, phosphorylation or carbonylation (Mann and Jensen 2003). Collectively, therefore, (bio)molecular technologies a) contribute significantly to the formulation of the profile in neurodegeneration, and b) constitute essential tools of research on brain and tissue samples originating in a reliable brain bank, thereby aiding advancements in diagnosis and potential treatments.
Objectives and Merit of a Greek Brain Bank
Presently, there is no organized brain and tissue bank operating in North Greece, because of the lack of technical infrastructure and paucity of brain donors. The main objectives of establishing and running such a brain bank should be based on criteria maintaining gold standards of excellence in brain banking, seeking archiving, and storing frozen and formalin-fixed tissue(s). In addition, the essential role for such a brain bank is to provide teaching, training skills and educational resources for neuroscientists, while concurrently facilitating (inter)national collaboration among research scientists, pathologists and clinicians through established bio-ethical guidelines. Apart from that, a well-organized brain bank should a) ensure optimum patient and donor care, and maintain contact with donor families, informing them about diagnosis and progress in research, and b) disseminate or promote knowledge of brain banking through scientific meetings, public fora and publications (Figure 2). For such a collective endeavor to succeed in North Greece there is an urgent need to establish and facilitate brain donation through an ethically approved process, preserve brain tissue in reliable infrastructure facilities, offer education, training and information, and provide pathological diagnosis to the relatives of brain donors, in line with the best national and international guidelines. To this end, carefully designed campaigns at the local and national level are to be launched a) emphasizing the importance of brain tissue donation programs embraced by local and national communities, and b) denoting the benefits of society from research promoted as a result of the availability of ample brain and tissue samples. Training of specialist health personnel, dissemination of knowledge, education, amply and readily understood information in schools, and wide publicity through the written Press and all electronic media stand to support the establishment and viability of such a brain bank, which further intends to a) benefit not only North Greece but also the abutting Balkan country communities, and b) hopefully contribute to the global community as an active member of an international network of brain banks (Donatelli et al. 2006; McKeown et al. 2012) .There is a brain bank in Athens, Greece, with which we will collaborate very closely.
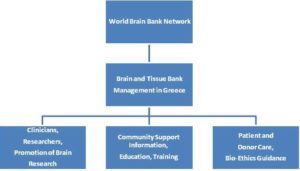
Fig. 2: The Principal Objectives for the Organization of Human Brain and Tissue Banking in North Greece and Connection to the Global Brain Bank Network
Establishment of the herein proposed brain bank aims at setting the foundations for a simple, efficient, nationwide system targeting brain donation. The initiative hopes to motivate more people to get involved in brain donation, an action that will in turn increase the number of brains available for this important research. The wider goal is to create a research-based brain bank, which will act as a reference center, accepting donated brain and tissue samples from all over Greece and many neighboring countries as well (Bulgaria, Albania, Romania, Serbia, etc.), where there is currently no such brain bank center in operation. This will promote brain research in the field of neurodegenerative diseases in Greece and provide the local and greater international scientific community with brain samples for research that will lead to advances in the treatment, cure and prevention of brain diseases and disorders. Moreover, the proposed brain bank is expected to provide information, teaching, training and educational resources for neuroscientists and patients through scientific meetings, public engagements and publications. To this end, the specific brain bank is expected to stand as a paradigm of a center for research and education in the entire Balkan region, progressively becoming a research pole of attraction and an active center in the worldwide brain research community.
Discussion
Brain Bank will help neuroscientists who participate in the attempt to gather genetic material from tissue and fluid samples of the brain. The study material will help, as they say, to answer questions about the prevention and treatment of neurological disorders that affects milllions of people and remain incurable.
The main goal of brain banks is to use the high quality postmortem brain tissue for research purposes and shed light to the cellular signaling pathways involved in neurological diseases (Cruz-Sánchez and Tolosa 1993). These disorders include mainly, Parkinson’s disease (Deep-Soboslay et al. 2011), the variant Creutzfeldt-Jakob Disease (vCJD) and Alzheimer’s disease (AD) (Rinne and Sonninen 1968) (Olney and Farber 1995) (Chalmers et al. 2009). Overall, the use of brain tissue samples has proven critical for researchers to study the neuronal pathway mechanisms involved in brain metabolism (Go et al. 2005).
In a global research setting, current obstacles for scientists emerge from lack of basic knowledge in neurological diseases, including Alzheimer’s and Parkinson’s diseases, multiple sclerosis, stroke, schizophrenia, bipolar disorder, and major depression. Gaining such knowledge is expected to lead to a better understanding of the abnormal mechanisms operating in these diseases and will, in turn, help develop new approaches for potential treatments (Ghosh et al. 2012). Through advances in technology, it is now possible to conduct large scale studies of genetic variability in gene expression control. This, however, requires high quality and well-characterized human brain tissue samples.
In view of the aforementioned grounds, the purpose of establishing such a brain and tissue bank in North Greece is to support multidisciplinary neuroscience research in the framework of a global brain-bank network, by providing appropriate tissue in an efficient and cost-effective manner, while meeting uniformly global, standardized, and high ethical and scientific standards of operation and accountability.
Conclusions
Over the last decade, genomics, proteomics, and research in neuroscience have helped scientists delineate key steps in the pathogenesis of three major neurodegenerative diseases: AD, HD, and PD. Justifiably, therefore, many researchers believe and hope that the cause of AD and PD will be discovered in the next 10 to 15 years. With this expectation in perspective, scientists think of a brain bank as the main bridge between patients and researchers. This groundbreaking type of organization could make it possible for basic scientists to probe, peruse and unravel the pathogenesis (or key elements thereof) of neurodegenerative diseases that can be used to design treatments, identify markers of active disease, and finally create diagnostic tests to encourage early clinical diagnosis. Undoubtedly, therefore, supporting the establishment of a brain and tissue bank in Greece provides a breakthrough opportunity for investigators to gain access to high quality human postmortem tissue to be collected, processed, and stored, all through globally standardized scientific and ethical criteria, for scientific multidisciplinary research, meriting tangible results for the benefit of a contemporary worldwide human society.
References
Ahmann, G. J., Chng, W. J., Henderson, K. J., Price-Troska, T. L., DeGoey, R. W., Timm, M. M., Dispenzieri, A., Greipp, P. R., Sable-Hunt, A., Bergsagel, L. & Fonseca, R. (2008). “Effect of Tissue Shipping on Plasma Cell Isolation, Viability, and RNA Integrity in the Context of a Centralized Good Laboratory Practice-Certified Tissue Banking Facility,”Cancer Epidemiology, Biomarkers & Prevention, 17(3):666-73.
Publisher – Google Scholar
Alafuzoff, I., Pikkarainen, M., Al-Sarraj, S., Arzberger, T., Bell, J., Bodi, L., Bogdanovic, N., Budka, H., Bugiani, O., Ferrer, I., Geipi, E., Giaccone, G., Graeber, M. B., Hauw, J. J., Kamphorst, W., King, A., Kopp, N., Korkolopoulou, P., Kovacs, C. G., Meyronet, D., Parchi, P., Patsouris, E., Preusser, M., Ravid, R., Roggendorf, W., Seilhean, D., Streichenberger, N., Thal, D. R. & Kretzschmar, H. (2006). “Interlaboratory Comparison of Assessments of Alzheimer Disease-Related Lesions: A Study of the Brainnet Europe Consortium,” Journal of Neuropathology and Experimental Neurology, 65(8):740—57.
Publisher – Google Scholar
Bathgate, D., Snowden, J. S., Varma, A., Blackshaw, A. & Neary, D. (2001). “Behaviour in Frontotemporal Dementia, Alzheimer’s Disease and Vascular Dementia,” Acta Neurologica Scandinavica 103(6):367-78.
Publisher – Google Scholar
Bayés, A. & Grant, S. G. N. (2009). “Neuroproteomics: Understanding the Molecular Organization and Complexity of the Brain,” Nature Reviews Neuroscience 10:635-46.
Publisher – Google Scholar
Beaulieu, A. (2004). “From Brain Bank to Database: The Informational Turn in the Study of the Brain,” Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences 35:367—90.
Publisher – Google Scholar
Bell, J. E. & Ironside, J. W. (1997). “Principles and Practice of ”High Risk” Brain Banking,” Neuropathology and Applied Neurobiology 23:281—88.
Publisher – Google Scholar
Bidaut-Russell, M., Ravid, R., Cruz-Sa’Nchez, F. F., Grossberg, G. T. & Mckeel, D. W. (1995). “Survey of North American and European Dementia Brain Banks: A 1994 Directory,” Alzheimer disease and Associated Disorders9(4):193—202.
Publisher – Google Scholar
Boller, F., Mizutani, T., Roessmann, U. & Gambetti, P. (1980). “Parkinson Disease, Dementia, and Alzheimer’s Disease: Clinicopathological Correlations,” Annals of Neurology 7:329 —35.
Publisher – Google Scholar
Cacabelos, R., Martinez-Bouza, R., Carril, J. C., Fernandez-Novoa, L., Lombardi, V., Carrera, I., Corzo, L. & Mckay, A. (2012). “Genomics and Pharmacogenomics of Brain Disorders,” Current Pharmaceutical Biotechnology 13:674-725.
Publisher – Google Scholar
Cambon-Thomsen, A. (2004). “The Social and Ethical Issues of Post-Genomic Human Bio Banks,” Nature Reviews Genetics 5(11):866—73.
Publisher – Google Scholar
Cassel, C. K. (1998). “Genetic Testing and Alzheimer’s Disease; Ethical Issues for Providers and Families,” Alzheimer disease and Associated Disorders 12(3):S16—20.
Publisher – Google Scholar
Celis, J. E., Ostergaard, M., Jensen, N. A., Gromova, I., Rasmussen, H. H. & Gromov, P. (1998). “Human and Mouse Proteomic Databases: Novel Resources in the Protein Universe,” FEBS Letters 430:64—72.
Publisher – Google Scholar
Chalmers, K. A., Wilcock, G. K., Vinters, H. V., Perry, E. K., Perry, R., Ballard, C. G. & Love, S. (2009). “Cholinesterase Inhibitors May Increase Phosphorylated Tau in Alzheimer’s Disease,” Journal of Neurology 256(5):717-20.
Publisher – Google Scholar
Cruz-Sánchez, F. F. & Tolosa, E. (Eds) (1993). ‘How to Run a Brain Bank,’ Journal of Neural Transmission S39:1—242.
Cruz-Sánchez, F. F., Ravid, R. & Cuzner, M. L. (1995). ‘The European Brain Bank Network (EBBN) and the Need of Standardized Neuropathological Criteria for Brain Tissue Cataloguing,’ In: Cruz-Sánchez FF, Cuzner ML, Ravid R (Eds) Neuropathological Diagnostic Criteria for Brain Banking. European Union Biomedical and Health Research, Vol 10. IOS Press, Amsterdam, Pp 1—3.
Google Scholar
Cruz-Sánchez, F. F., Mordini, E. & Ravid, R. (1997). “Ethical Aspects to be Considered in Brain Banking,” Annali Dell’ Lstituto Superiore di Sanità 33:477—82.
Publisher – Google Scholar
Daneshtalab, N., Doré, J. J. & Smeda, J. S. (2010). “Troubleshooting Tissue Specificity and Antibody Selection: Procedures in Immunohistochemical Studies,” Journal of Pharmacological and Toxicological Methods 61:127-35.
Publisher – Google Scholar
Deep-Soboslay, A., Benes, F. M., Haroutunian, V., Ellis, J. K., Kleinman, J. E. & Hyde, T. M. (2011). “Psychiatric Brain Banking: Three Perspectives on Current Trends and Future Directions,” Biological Psychiatry 69(2):104—12.
Publisher – Google Scholar
Dodd, P. R., Hambley, J. W., Cowburn, R. F. & Hardy, J. A. (1988). “A Comparison of Methodologies for the Study of Functional Transmitter Neurochemistry in Human Brain,” Journal of Neurochemistry 50:1333— 45.
Publisher – Google Scholar
Donatelli, L. A., Geocadin, R. G. & Williams, M. A. (2006). “Ethical Issues in Critical Care and Cardiac Arrest: Clinical Research, Brain Death, and Organ Donation,” Seminars in Neurology 26:452-9.
Publisher – Google Scholar
Du, A. T., Schuff, N., Laakso, M. P., Zhu, X. P., Jagust, W. J., Yaffe, K., Kramer, J. H., Miller, B. L., Reed, B. R., Norman, D., Chui, H. C. & Weiner, M. W. (2002). “Effects of Subcortical Ischemic Vascular Dementia and AD on Entorhinal Cortex and Hippocampus,” Neurology 58:1635—41.
Publisher – Google Scholar
Dudley, E., Häßler, F. & Thome, J. (2011). “Profiling for Novel Proteomics Biomarkers in Neurodevelopmental Disorders,” Expert Review of Proteomics Vol. 8, No. 1, Pp. 127-36.
Publisher – Google Scholar
Duyckaerts, C., Sazdovitch, V., Seilhean, D., Delaère, P. & Hauw, J. J. (1993). “A Brain Bank in a Neuropathology Laboratory (With Some Emphasis on Diagnostic Criteria),” Journal of Neural Transmission. Supplementa 39:107— 18.
Publisher – Google Scholar
Esiri, M. M., Wilcock, G. K. & Morris, J. H. (1997). “Neuropathological Assessment of the Lesions of Significance in Vascular Dementia,” Journal of Neurology, Neurosurgery and Psychiatry 63:749—53.
Publisher – Google Scholar
Ferrer, I., Martinez, A., Boluda, S., Parchi, P. & Barrachina, M. (2008). “Brain Banks: Benefits, Limitations and Cautions Concerning the Use of Post-Mortem Brain Tissue for Molecular Studies,” Cell and Tissue Banking 3:181-94.
Publisher – Google Scholar
Ferrer, I., Santpere, G., Arzberger, T., Bell, J., Blanco, R., Boluda, S., Budka, H., Carmona, M., Giaccone, G., Krebs, B., Limido, L., Parchi, P., Puig, B., Strammiello, R., Ströbel, T. & Kretzschmar, H. (2007). “Brain Protein Preservation Largely Depends on the Postmortem Storage Temperature: Implications for Study of Proteins in Human Neurologic Diseases and Management of Brain Banks: A Brainnet Europe Study,” Journal of Neuropathology and Experimental Neurology 66:35—46.
Publisher – Google Scholar
Fountoulakis, M. (2001). “Proteomics: Current Technologies and Applications in Neurological Disorders and Toxicology,”Amino Acids 21:363—81.
Publisher – Google Scholar
Gillardon, F., Rist, W., Kussmaul, L., Vogel, J., Berg, M., Danzer, K., Kraut, N. & Hengerer, B. (2007). “Proteomic and Functional Alterations in Brain Mitochondria from Tg2576 Mice Occur before Amyloid Plaque Deposition,” Proteomics7:605—16.
Publisher – Google Scholar
Gindro, S. & Mordini, E. (1998). ‘Ethical, Legal and Social Issues in Brain Research,’ Current opinion in Psychiatry11(5):575—80.
Google Scholar
Go, R. C. P., Perry, R. T., Wiener, H., Bassett, S. S., Blacker, D., Devlin, B. & Sweet, R. A. (2005). “Neuregulin-1 Polymorphism in Late Onset Alzheimer’s disease Families with Psychoses,” American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 139B(1):28-32.
Publisher – Google Scholar
Hakimian, R. & Korn, D. (2004). “Ownership and Use of Tissue Specimens for Research,” JAMA 292:2500-5.
Publisher – Google Scholar
Hanyu, H. (2012). “Diagnosis and Treatment of Mixed Dementia,” Brain and Nerve= Shinkei Kenkyū no Shinpo 64:1047-55.
Publisher – Google Scholar
Hasson, J. & Schneiderman, H. (1995). “Autopsy Training Programs: To Right a Wrong,” Archives of Pathology & Laboratory Medicine 119:289—91.
Publisher – Google Scholar
Hauptmann, S., Scherping, I., Drose, S., Brandt, U., Schulz, K. L., Jendrach, J., Leuner, K., Eckert, A. & Müller, W. E. (2009). “Mitochondrial Dysfunction: An Early Event in Alzheimer Pathology Accumulates with Age in AD Transgenic Mice,” Neurobiology of Aging 30:1574—86.
Publisher – Google Scholar
Holmes, C., Cairns, N., Lantos, P. & Mann, A. (1999). “Validity of Current Clinical Criteria for Alzheimer’s Disease, Vascular Dementia and Dementia with Lewy Bodies,” The British Journal of Psychiatry 174:45—50.
Publisher – Google Scholar
Jellinger, K. A., Lantos, P. L. & Mehraein, P. (1993). “Pathological Assessment of Movement Disorders: Requirements for Documentation in Brain Banks,” Journal of Neural Transmission. Supplementum 39:173—84.
Publisher – Google Scholar
Katelaris, A., Kencian, J., Duflou, J. & Hilton, J. M. N. (1994). “Brain at Necropsy: To Fix or Not to Fix?,” Journal of Clinical Pathology 47:718—20.
Publisher – Google Scholar
Kearney, J. N. (1998). “Quality Issues in Skin Banking: A Review,” Burns 24:299—305.
Publisher – Google Scholar
Kiehntopf, M., Siegmund, R. & Deufel, T. (2007). “Use of SELDI-TOF Mass Spectrometry for Identification of New Biomarkers: Potential and Limitations,” Clinical Chemical Laboratory Medicine 45:1435—49.
Publisher – Google Scholar
Knopman, D. S., Boeve, B. F. & Petersen, R. C. (2003). “Essentials of the Proper Diagnoses of Mild Cognitive Impairment, Dementia, and Major Subtypes of Dementia,” Mayo Clinic Proceedings 78:1290-308.
Publisher – Google Scholar
Korolainen, M. A., Nyman, T. A., Aittokallio, T. & Pirttilä, T. (2010). “An Update on Clinical Proteomics in Alzheimer’s Research,” Journal of Neurochemistry 12:1386-414.
Publisher – Google Scholar
Kretzschmar, H. (2009). “Brain Banking: Opportunities, Challenges and Meaning for the Future,” Nature Reviews Neuroscience 10:70-8.
Publisher – Google Scholar
Lopez, O. L., Litvan, I., Catt, K. E., Stowe, R., Klunk, W., Kaufer, D. I., et al. (1999). “Accuracy of Four Diagnostic Criteria for the Diagnosis of Neurodegenerative Dementias,” Neurology 53:1292—9.
Publisher – Google Scholar
Mann, M. & Jensen, O. N. (2003). “Proteomic Analysis of Post-Translational Modifications,” Nature Biotechnology21(3):255-61.
Publisher – Google Scholar
Mckeown, D. W., Bonser, R. S. & Kellum, J. A. (2012). “Management of the Heartbeating Brain-Dead Organ Donor,”British Journal of Anaesthesia 108:96-107.
Publisher – Google Scholar
Mendez, M. F., Ghajarania, M. & Perryman, K. M. (2002). “Posterior Cortical Atrophy: Clinical Characteristics and Differences Compared to Alzheimer’s Disease,” Dementia and Geriatric Cognitive Disorders 14:33—40.
Publisher – Google Scholar
Micheva, K. D. & Bruchez, M. P. (2012). “The Gain in Brain: Novel Imaging Techniques and Multiplexed Proteomic Imaging of Brain Tissue Ultrastructure,” Current Opinion in Neurobiology 22:94-100.
Publisher – Google Scholar
Morris, J. C. (1993). “The Clinical Dementia Rating (CDR): Current Version and Scoring Rules,” Neurology 43:2412— 4.
Publisher – Google Scholar
Mufson, E. J., Binder, L., Counts, S. E., Dekosky, S. T., De Toledo-Morrell, L., Ginsberg, S. D., Ikonomovic, M. D., Perez, S. E. & Scheff, S. W. (2012). “Mild Cognitive Impairment: Pathology and Mechanisms,” Acta Neuropathologica123:13-30.
Publisher – Google Scholar
Neary, D., Snowden, J. S. & Mann, D. M. (2000). “Classification and Description of Frontotemporal Dementias,” Annals of the New York Academy of Sciences 920:46-51.
Publisher – Google Scholar
O’Farrell, P. H. (1975). “High Resolution Two-Dimensional Electrophoresis of Proteins,” Journal of Biological Chemistry250:4007—21.
Publisher – Google Scholar
Oddo, S., Caccamo, A., Kitazawa, M., Tseng, B. P. & Laferla, F. M. (2003). “Amyloid Deposition Precedes Tangle Formation in a Triple Transgenic Model of Alzheimer’s Disease,” Neurobiology of Aging 24:1063—70.
Publisher – Google Scholar
Olney, J. W. & Farber, N. B. (1995). “Glutamate Receptor Dysfunction and Schizophrenia,” Archives of General Psychiatry 52(12):998-1007.
Publisher – Google Scholar
Perry, E. K. & Perry, R. H. (1983). “Human Brain Neurochemistry, Some Postmortem Problems,” Life Sciences33:1733—43.
Publisher – Google Scholar
Ravid, R. & Grinberg, L. T. (2008). “How to Run a Brain Bank-Revisited,” Cell and Tissue Banking 9:149-50.
Publisher – Google Scholar
Reiber, H. (1994). “Flow-Rate of Cerebrospinal-Fluid (CSF) — A Concept Common to Normal Blood—CSF Barrier Function and to Dysfunction in Neurological diseases,” Journal of the Neurological Sciences 122:189—203.
Publisher – Google Scholar
Rinne, U. K. & Sonninen, V. (1968). “A Double Blind Study of 1-Dopa Treatment in Parkinson’s Disease,” European Neurology 1(3):180-91.
Publisher – Google Scholar
Sposny, A. & Hewitt, P. G. (2012). “SELDI-TOF Mass Spectrometry-Based Protein Profiling of Tissue Samples for Toxicological Studies,” Methods in Molecular Biology, Volume 818, 119-129.
Publisher – Google Scholar
Stoop, M. P., Coulier, L., Rosenling, T., Shi, S., Smolinska, A. M., Buydens, L., Ampt, K., Stingl, C., Dane, A., Muilwijk, B., Luitwieler, R. L., Sillevis Smitt, P. A., Hintzen, R. Q., Bischoff, R., Wijmenga, S. S., Hankemeier, T., Van Gool, A. J. & Luider, T. M. (2010). “Quantitative Proteomics and Metabolomics Analysis of Normal Human Cerebrospinal Fluid Samples,” Molecular and Cellular Proteomics 9(9):2063-75.
Publisher – Google Scholar
Stuss, D. T. & Levine, B. (1996). “The Dementias: Nosological and Clinical Factors Related to Diagnosis,” Brain and Cognition 31:99 —113.
Publisher – Google Scholar
Suh, K. S., Remache, Y. K., Patel, J. S., Chen, S. H., Haystrand, R., Ford, P., Shaikh, A. M., Wang, J. & Goy, A. H. (2009). “Informatics-Guided Procurement of Patient Samples for Biomarker Discovery Projects in Cancer Research,”Cell and Tissue Banking 10:43-8.
Publisher – Google Scholar
Tumani, H., Lehmensiek, V., Lehnert, S., Otto, M. & Brettschneider, J. (2010). “2D DIGE of the Cerebrospinal Fluid Proteome in Neurological Diseases,” Expert Review of Proteomics 7:29—38.
Publisher – Google Scholar
Verghese, J., Lipton, R. B., Hall, C. B., Kuslansky, G., Katz, M. J. & Buschke, H. (2002). “Abnormality of Gait as a Predictor of Non-Alzheimer’s Dementia,” The New England Journal of Medicine 347:1761—8.
Publisher – Google Scholar
Von Versen, R. (1999). “Musculoskeletal Tissue Banking in Europe, Regulations and Quality Assurance,” Annales Chirurgiae et Gynaecologiae 88: 215—20.
Publisher – Google Scholar
Vonsattel, J.- P. G., Aizawa, H., Ge, P., Difiglia, M., Mckee, A. C., Macdonald, M., Gusella, J. F., Landwehrmeyer, B., Bird, E. D., Richardson, E. P. Jr, & Hedley-Whyte, E. T. (1995). “An Improved Approach to Prepare Human Brains for Research,” Journal of Neuropathology and Experimental Neurology 54:42—56.
Publisher – Google Scholar
Ward, M., Güntert, A., Campbell, J. & Pike, I. (2009). “Proteomics for Brain Disorders–The Promise for Biomarkers,”Annals of the New York Academy of Sciences 11(80):68-74.
Publisher – Google Scholar
Weiss, W., Weiland, F. & Gorg, A. (2009). “Protein Detection and Quantitation Technologies for Gel-Based Proteome Analysis,” Methods in Molecular Biology 564:59—82.
Publisher – Google Scholar
Xu, J., Chen, J., Peskind, E. R., Jin, J., Eng, J., Pan, C., Montine, T. J., Goodlett, D. R. & Zhang, J. (2006). “Characterization of Proteome of Human Cerebrospinal Fluid,” International Review of Neurobiology 73:29—98.
Publisher – Google Scholar
Yao, J., Irwin, R. W., Zhao, L., Nilsen, J., Hamilton, R. T. & Brinton, R. D. (2009). “Mitochondrial Bioenergetic Deficit Precedes Alzheimer’s Pathology in Female Mouse Model of Alzheimer’s Disease,” Proceedings of the National Academy of Sciences of the United States of America 106:14670—5.
Publisher – Google Scholar
Yuan, X. L. & Desiderio, D. M. (2005). “Human Cerebrospinal Fluid Peptidomics,” Journal of Mass Spectrometry40:176—81.
Publisher – Google Scholar
Zhang, J., Goodlett, D. R., Peskind, E. R., Quinn, J. F., Zhou, Y., Wang, Q., Pan, C., Yi, E., Eng, J., Aebersold, R. H. & Montine, T. J. (2005). “Quantitative Proteomic Analysis of Age-Related Changes in Human Cerebrospinal Fluid,”Neurobiology of Aging 26:207—27.
Publisher – Google Scholar




