Introduction
Stroke is the leading cause of adult disability in Western countries, and the second cause of cardiovascular mortality with high economic and social impact [1], [2]. One third of the strokes are recurrent attacks [3]. The total number of stroke deaths is currently estimated at 508,000 per year in Europe [4]. Ischemic stroke is the most frequent etiology (87%) [3].
The cumulative risk of stroke recurrence within five years after a first episode ranges between 15-40%[5,6]. The risk of recurrence is higher within the first year after the stroke (between 6-14%) than in subsequent years (4% annually), achieving its maximum incidence during the first month after the initial stroke.
The most relevant predictors of stroke recurrence identified in epidemiological trials include advancing age [9,10,12], arterial hypertension [12,14,15], a trial fibrillation [15,16], diabetes mellitus (DM) or impaired glucose tolerance [5,10,12,17], hyperlipidemia [15] and previous transient ischemic attack [5,16, 18].
There are still variability between studies that based in data drawn from point of care versus epidemiological settings, particularly in the role of long term medical treatment [19]. The ability to predict the risk of stroke recurrence through a clinical prediction algorithm would help to tailor future preventive and therapeutic measures according to the estimated risk of recurrence for each individual patient. We hypothesized that it will facilitate the decision-making process and long-term strategies for stroke’s secondary prevention. Thus, the main objective of this study was to develop a practical and user-friendly clinical prediction algorithm of ischemic stroke recurrence in patients admitted to the hospital after a first episode of ischemic stroke.
Materials and Methods
Study Design
This observational, historical cohort study was conducted at the University Hospital 12 de Octubre, Madrid (Spain). Study subjects were recruited throughout a three-year period, from October 1998 until October 2001, both years inclusive. The study was approved by the Ethics Committee of the hospital and performed in accordance with Good Clinical Practices criteria.
The mean reference population for University Hospital 12 Octubre was numbered 550,000 by the year 2000. This hospital was the only one that attended acute strokes in their geographical area and most of the stroke patients were admitted to Internal Medicine and Neurology Departments (according beds availability).
Study Procedures
Patients older than 15 years old, admitted to the emergency room with the first episode of ischemic stroke (transient ischemic attack included) were identified from the hospital’s database and included in the study
Clinical information during hospitalization was obtained from inpatient medical records and follow-up information through outpatient medical records or by telephone contact with the patient or next of kin.
Ischemic stroke was defined as a cerebrovascular event that rapidly led to the development of clinical signs of acute neurological focal disturbance, or leading to death with no apparent cause other than vascular origin. Recurrent stroke was defined as a cerebrovascular event subsequent to the initial stroke and occurring in an anatomic site or vascular territory different from that of the initial stroke. Diagnosis of ischemic stroke (initial or recurrent) was done based on inpatient and outpatient medical records and computed tomography or magnetic resonance imaging scan reports. Cranial CT scan was performed in all patients included in the study. Doppler sonography of cervical (vertebral and carotid) arteries was performed in 72%, echocardiography in 41% and cerebral MRI in 39%. We defined Hypertensive Cardiomyopathy as the presence of conventional electrocardiographic and/or ultrasonographic criteria for left ventricular hypertrophy and/or for diastolic dysfunction.
Protocol study included the following patient’s related information: demographic and clinical data, previous vascular risk factors (VRF) such as HTA, smoker status, diabetes, atrial fibrillation, cardiovascular events or antecedents of ischemic heart disease, hypertensive cardiomyopathy, left ventricular hypertrophy based on conventional electrocardiographic or ultrasonographic criteria. Ischemic stroke subtype was classified based on TOAST classification criteria [20] and severity of stroke at discharge based on RANKIN INDEX [21], (scale that assesses the degree of physical disability after stroke). Also degree of control of cardiovascular risk factors after first ever ischemic stroke. We considered non-optimal control -uncontrolled arterial hypertension- if there were in the patient clinical record more than 2 documented BP measures above 140/90 mmHg or more than 2 references of inadequate BP control, non-optimalglycemic control if A1c hemoglobin > 7%, non-optimal lipid profile control if LDL cholesterol above 100 mg/dl; new cardiovascular events during follow-up (stroke, acute coronary heart disease), and therapy: anticoagulant and antiplatelet drugs among others.
Statistical Analysis
Qualitative variables were expressed as absolute frequencies and percentages. Continuous variables were described using mean and standard deviation.
Significant risk factors for ischemic stroke recurrence were identified using a univariate analysis. Then, a multivariate analysis of significant risk factors of ischemic stroke identified in the univariate analysis, as well as other risk factors that we considered clinically relevant, was performed using the Cox proportional hazards model.
If death and a possible recurrent ischemic stroke were recorded as occurring simultaneously, only the death was considered as a censoring event because of the difficulty of documenting the ischemic stroke recurrence at that precise moment. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each risk factor. Lastly, clinically relevant risk factors of ischemic stroke recurrence were subjected to a classification and regression tree analysis. Only patients who had all the necessary information to assign the best documented and validated score were included in this analysis.This recursive partitioning method was used for the multivariate analysis due to its ability to identify groups with similar prognosis, place the different prognostic factors in a hierarchy, and demonstrate the relationships between them. The recursive partitioning method may reutilize the same variable in different segments of the tree. In each segment, the most convenient cut off-point that optimizes the partition criterion is chosen. Continuous variables were dichotomized to create two groups with different probability of event.
The prediction models were estimated regarding recurrence during the first two years given that is the time spam with more information and sample size. The area under the receiver operating characteristic (ROC) curve was calculated to evaluate the performance (predictive ability) of the Cox regression and classification and regression model. Finally, the areas under the ROC curves obtained in each model were compared to identify any differences regarding the discrimination power between the models.
The internal validity of the model’s estimations was calculated via bootstrapping. 200 bootstrap samples were needed for accurate assessment of outer confidence limits.
All statistical analyses were carried out using STATA V 11 software. Significance was set at p<0.05.
Ethical approval: The proposal for the project was approved as observational study by the Institutional Review Board (ref. 149/05) in compliance with the Helsinki Declaration and BPC (CPMP/ICH/135/95).
Results
During the 3-year inclusion period (1998-2001), 449 patients with a first-ever ischemic stroke were identified. Of these, 35 were excluded due to insufficient clinical data, 37 because they died of the initial stroke and 52 because they could not be followed up after hospital discharge. Thus, 325 patients were finally included and followed up according to the study protocol.
Mean age of study subjects was 70±12 years, and 56% were males. Those patients included had an averaged historical follow up of 41±34 months until closing the data set for this study. Baseline patient characteristics are shown in table 1.
Table 1: Baseline Patient Characteristics (N=325)
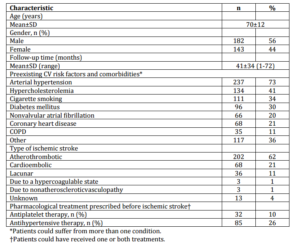
ACEI: Angiotensinconverterenzymeinhibitors. CI: confidence interval. COPD: Chronic obstructivepulmonary disease. CV: Cardiovascular. SD: Standard deviation.
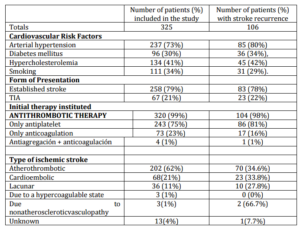
During follow-up, 56 patients died (17%) (21 of them died of stroke recurrence, 19 of other cardiovascular events, and 16 of non-vascular disease). Ischemic stroke recurrence during follow-up, including stroke deaths were of 33%.
In patients with stroke recurrence, only 3% were younger than 50 years while 43% were older than 75 years old.
Of the 106 patients who suffered from an ischemic stroke recurrence, 45 patients (43%) suffering in the first year, 29 (27%) during the second year, 19 (18%) during the third year, and 13 patients (12%) during the following years.
Table 2 shows the characteristics of patients with stroke-recurrence.
Table 2: Characteristics of Patients with Stroke Recurrence
Significant risk factors of ischemic stroke recurrence after a first episode identified with univariate analysis were: age (Hazard ratio —HR-: 1.038; p<0.001), chronic kidney disease (HR: 2.47 p=0.01), coronary heart disease (HR: 1.841; p=0.004), hypertensive cardiomyopathy/left ventricular hypertrophy (HR: 2.127;p=0.01), uncontrolled arterial hypertension (HR: 2.160;p=0.025) and stroke despite previous antiplatelet therapy (HR: 2.037; p=0.002). However, starting oral anticoagulants (HR: 0.483; p=0.007) was identified as factor associated with lesser risk of ischemic stroke recurrence.
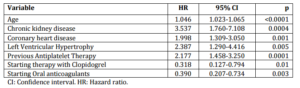
The variables identified in the multivariate analysis were: age (HR: 1.046; p<0.0001), chronic kidney disease (HR: 3.537; p=0.0004), ischemic heart disease (HR: 1.998; p=0.0013), hypertensive cardiomyopathy/LVH (HR: 2.387; p=0.0056) and previous antiplatelet therapy (HR: 2.177; p=0.0001) (Table 3). Starting therapy with oral anticoagulants (HR: 0.390; p=0.003) and clopidogrel use (HR: 0.318; p=0.01) were associated with a lower recurrence.
Table 3: Cox Multivariate Analysis of Predictive Risk Factors of Ischemic Stroke Recurrence (N=325)
The area under the ROC curve for the multivariate Cox analysis was 0.7495 (95% CI: 0.675-0.803), bootstrap validation confirm the size of the area under the curve again (bias-corrected 95% CI: 0.671-0.800), indicating a good predictive power (Figure 1).
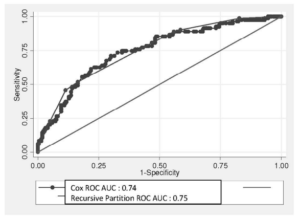
Figure 1: Comparison of Areas under the ROC Curve Obtained in the Cox and Recursive Partition Algorithm Analyses
Recursive partition and regression classification algorithm analysis for clinical prediction rule, by this method, only 303 patients fulfilled all requirements. The best single predictor of stroke recurrence in the first 2 years was being older 70 years old.
The major independent predictor of stroke recurrence in patients elder than 70 years old was the presence of hypertensive cardiomiopathy or left ventricular hypertrophy. However, anticoagulant therapy was clearly protective factor.
Table 4 shows the final recursive partition algorithm analysis together with the probability of stroke recurrence, the number of patients, and the clinical characteristics for the four risk groups were identified by the model.
Table 4: Clinical Prediction Rule. Clinical Predictors of 2-Year Stroke Recurrence after Recursive Partition Algorithm Analysis
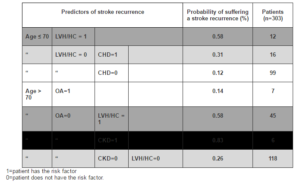
LVH/HC: Left ventricular hypertrophy/ hypertensive cardiomyopathy; CHD: Coronary heart disease; OA: start oral anticoagulants; CKD: chronic kidney disease.
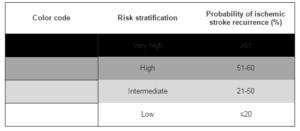
From the 303 patients studied, 6 of them (2%) were classified as having a ‘very high’ risk, 57 (19%) as ‘high’ risk, 134 (44%) as ‘intermediate’ risk, and 106 (35%) as ‘low’ risk of ischemic stroke recurrence.
According to the recursive partition algorithm analysis (table 3), patients with left ventricular hypertrophy/hypertensive cardiomyopathy or chronic kidney disease were classified as a “high” or “very high” risk of ischemic stroke recurrence. In other hand, the patients elder than 70 years old whose starting anticoagulants therapy, and younger than 70 years old without any pathology were considered low risk.
Note that some logical clinical profiles expected were absent due to the lack of enough subjects in the data set with these characteristics. The area under the ROC curve of the recursive partition algorithm analysis was 0.7557 (95% CI: 0.697-0.813), (bootstrap bias-corrected 95% CI: 0.688-0.804) demonstrating a good predictive ability. When the areas under the ROC curve obtained in the Cox regression analysis and the recursive partition algorithm analysis were compared, no significant differences were observed regarding the ability to predict a stroke recurrence after a first episode (p=0.827) (Figure 2).
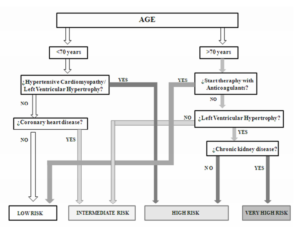
Figure 2: Algorithm for Easily Stratification Stroke Recurrence Risk
Discussion
Stroke is a major public health problem, and in particular ischemic stroke is the subtype that generates the greatest burden of disease [22] Thus, the development of practical and user-friendly clinical prediction algorithms to rapidly and accurately estimate the patient’s short and long-term risk of suffering a stroke recurrence is both timely and necessary.
Our results showed that after a mean follow-up period of 41 months, the third part (33%) of the patients had suffered from an ischemic stroke recurrence; moreover, 6% of patients died as a consequence of the recurrence.
These results underscore the fact that, although the incidence of stroke recurrence varies depending on stroke subtypes, study design and other controlled and uncontrolled factors, it is high and must be addressed in time to avoid recurrence-related morbidity and mortality.
The predictive factors used in our study to develop clinical prediction rule for recurrent ischemic stroke, have already been described in previous studies. [10, 14, 15, 25-27].According to our results, age (p<0.0001), chronic kidney disease (p=0.01), coronary heart disease (p=0.004), hypertensive cardiomyopathy/ left ventricular hypertrophy (p=0.01) and uncontrolled arterial hypertension (p=0.025) were significant predictors of ischemic stroke recurrence after a first episode. Starting therapy with oral anticoagulants (in patients with demonstration of underlying disease with indication for it) (p=0.007) and the use of clopidogrel (p=0.01) were significant predictors of lower risk of recurrence.
There is enough scientific evidence to support the finding that advancing age is associated with an increased risk of stroke recurrence [10,14,24,25].
The American Heart Association report described that, patients who have a first-ever stroke of any type, in those aged 40 to 69 years 13% of men and 22% of women will have a stroke recurrence within the first five years after the first episode.
This incidence increases to 23% in men and 28% in women aged 70 years or more.
In a Mediterranean country as Spain, Modrego et al, showed that age, arterial hypertension, Diabetes mellitus and cardiovascular diseases, were significant predictors of stroke recurrence in 425 patients with a first episode of stroke. [9]
Arterial hypertension is a recognized risk factor for cardiovascular and cerebrovascular disease[28]related with atherosclerosis [29-32]. It is known that optimal control of blood pressure reduces the risk [33]as well as the recurrence of ischemic stroke[34,35].
We showed that hypertensive cardiomyopathy/ left ventricular hypertrophy and un-controlled arterial hypertension were strong predictive factors for stroke recurrence.
Although cardioembolic and atherothrombotic stroke have been linked to increased risk of recurrence in some studies[36,37] in our study, as in others [38],there was no difference in recurrence between different etiological subtypes of ischemic stroke.
We also observed that patients in treatment with antiplatelet therapy before first episode of stroke had more likelihood of recurrence. It could be explained by the presence of some illness (for instance a trial fibrillation, coronary heart disease, peripheral artery disease…), with potential added risk in these patients.
As it is well known, clopidogrel is an effective antiplatelet used for primary and secondary prevention of cardiovascular and cerebrovascular disease [39-43]. We noted that starting therapy with clopidogrel after a first-ever ischemic stroke was identified as a protective factor for stroke recurrence.
We observed the same results with anticoagulants therapy.
Thus, we proposed a useful algorithm based on the combination of 5 clinical parameters (figure 2), that facilitates the physician to classify the stroke recurrence risk of the patient into one of the four risk subgroups (very high, high, intermediate and low risk).
With this simple algorithm, the physician’s assessment of patients with a first stroke episode admitted to the hospital may be substantially enhanced, improving and facilitating the decision making process and course of action at the point of care, [44] and thereby with potential to slow down the morbidity and mortality of this prevalent entity.
Acknowledgements
We appreciate the contribution of Ximena Rodriguez (HealthCO), Berta Herrera Hueso and Pilar Cancelas in helping the writing of the manuscript.
References
[1] Di Carlo, A. (2009). “Human and Economic Burden of Stroke,” Age Ageing, 38: 4-5
Publisher – Google Scholar
[2] WHO. (2004).”The Global Burden of Disease: 2004 Update,” In, World Health Organization
Publisher – Google Scholar
[3] Lloyd-Jones, D., Adams, R., Carnethon, M., De Simone, G., Ferguson, T. B., Flegal, K., Ford, E., Furie, K., Go, A., Greenlund, K., Haase, N., Hailpern, S., Ho, M., Howard, V., Kissela, B., Kittner, S., Lackland, D., Lisabeth, L., Marelli, A., McDermott, M., Meigs, J., Mozaffarian, D., Nichol, G., O’Donnell, C., Roger, V., Rosamond, W., Sacco, R., Sorlie, P., Stafford, R., Steinberger, J., Thom, T., Wasserthiel-Smoller, S., Wong, N., Wylie-Rosett, J. & Hong, Y. (2009). “Heart Disease and Stroke Statistics-2009 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee,” Circulation, 119:e21-181.
Publisher – Google Scholar
[4] ‘European Cardiovascular Disease Statistics,’ (2008). In Brussels, European Heart Network, 2008.
[5] Burn, J., Dennis, M., Bamford, J., Sandercock, P., Wade, D. & Warlow, C. (1994). “Long-Term Risk of Recurrent Stroke after a First-Ever Stroke. The Oxfordshire Community Stroke Project,” Stroke, 25:333-337.
Publisher – Google Scholar
[6] Hardie, K., Jamrozik, K., Hankey, G. J., Broadhurst, R. J. & Anderson, C. (2005). “Trends in Five-Year Survival and Risk of Recurrent Stroke after First-Ever Stroke in the Perth Community Stroke Study,” Cerebrovasc Dis, 19:179-185.
Publisher – Google Scholar
[7] Hier, D. B., Foulkes, M. A., Swiontoniowski, M., Sacco, R. L., Gorelick, P. B., Mohr, J. P., Price, T. R. & Wolf, P. A. (1991). “Stroke Recurrence within 2 Years after Ischemic Infarction,” Stroke, 22:155-161.
Publisher – Google Scholar
[8] Sacco, R. L., Wolf, P. A., Kannel, W. B. & McNamara, P. M. (1982). “Survival and Recurrence Following Stroke. The Framingham Study,” Stroke, 13:290-295.
Publisher – Google Scholar
[9] Modrego, P. J., Mainar, R. & Turull, L. (2004). “Recurrence and Survival after First-Ever Stroke in the Area of Bajo Aragon, Spain. A Prospective Cohort Study,” Journal of the Neurological Sciences, 224:49-55.
Publisher – Google Scholar
[10] Lee, A. H., Somerford, P. J. & Yau, K. K. (2004). ‘Risk Factors for Ischaemic Stroke Recurrence after Hospitalization,’ Med J Aust, 181:244-246
[11] Petty, G. W., Brown, R. D., Jr., Whisnant, J. P., Sicks, J. D., O’Fallon, W. M. & Wiebers, D. O. (1998). “Survival and Recurrence after First Cerebral Infarction: A Population-Based Study in Rochester, Minnesota, 1975 through 1989,”Neurology, 50:208-216.
Publisher – Google Scholar
[12] Sacco, R. L., Shi, T., Zamanillo, M. C. & Kargman, D. E. (1994). “Predictors of Mortality and Recurrence after Hospitalized Cerebral Infarction in an Urban Community: The Northern Manhattan Stroke Study,” Neurology, 44:626-634.
Publisher – Google Scholar
[13] Rosamond, W. D., Folsom, A. R., Chambless, L. E., Wang, C. H., McGovern, P. G., Howard, G., Copper, L. S. & Shahar, E. (1999). “Stroke Incidence and Survival among Middle-Aged Adults: 9-Year Follow-up of the Atherosclerosis Risk in Communities (ARIC) Cohort,” Stroke, 30:736-743.
Publisher – Google Scholar
[14] Lai, S. M., Alter, M., Friday, G. & Sobel, E. (1994). “A Multifactorial Analysis of Risk Factors for Recurrence of Ischemic Stroke,” Stroke, 25:958-962.
Publisher – Google Scholar
[15] Leoo, T., Lindgren, A., Petersson, J. & von Arbin, M. (2008). “Risk Factors and Treatment at Recurrent Stroke Onset: Results from the Recurrent Stroke Quality and Epidemiology (RESQUE) Study,” Cerebrovascular Diseases, 25:254-260.
Publisher – Google Scholar
[16] Jorgensen, H. S., Nakayama, H., Reith, J., Raaschou, H. O. & Olsen, T. S. (1997). “Stroke Recurrence: Predictors, Severity, and Prognosis. The Copenhagen Stroke Study,” Neurology, 48:891-895.
Publisher – Google Scholar
[17] Prencipe, M., Culasso, F., Rasura, M., Anzini, A., Beccia, M., Cao, M., Giubilei, F. & Fieschi, C. (1998). “Long-Term Prognosis after a Minor Stroke: 10-Year Mortality and Major Stroke Recurrence Rates in a Hospital-Based Cohort,”Stroke, 29:126-132.
Publisher – Google Scholar
[18] Purroy, F., Jiménez Caballero, P. E., Gorospe, A., Torres, M. J., Alvarez-Sabin, J., Santamarina, E., Martínez-Sánchez, P., Cánovas, D., Freijo, M. M., Egido, J. A., Girón, J. M., Ramírez-Moreno, J. M., Alonso, A., Rodríguez-Campello, A., Casado, I., Delgado-Medeiros, R., Martí-FÃ bregas, J., Fuentes, B., Silva, Y., Quesada, H., Cardona, P., Morales, A., de la Ossa, N., García-Pastor, A., Arenillas, J. F., Segura, T., Jiménez, C., Masjuán, J., Stroke Proyect of the Spanish Cerebrovascular Diseases Study Group. (2012). “Prediction of Early Stroke Recurrence in Transient Ischemic Attack Patients from the PROMAPA Study: A Comparison of Prognostic Risk Scores,” Cerebrovascular Diseases, 33(2):182-189. [Epub ahead of print)
Publisher – Google Scholar
[19] Nilanont, Y., Komoltri, C., Saposnik, G., Côté, R., Di Legge, S., Jin, Y., Prayoonwiwat, N., Poungvarin, N. & Hachinski V. (2010). “The Canadian Neurological Scale and the NIHSS: Development and Validation of a Simple Conversion Model,” Cerebrovascular Diseases, 30(2):120-6. Epub 2010 May 22.PMID:20501997.
Publisher – Google Scholar
[20] Adams, H. P. Jr., Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., Gordon, D. L. & Marsh, E. E. erd. (1993). “Classification of Subtype of Acute Ischemic Stroke: Definitions for Use in a Multicenter Clinical Trial: TOAST: Trial of 10172 in Acute Stroke Treatment,” Stroke, 24:35-41.
Publisher – Google Scholar
[21] Hong, K. S. & Saver, J. L. (2009). “Quantifying the Value of Stroke Disability Outcomes. Who Global Burden of Disease Project Disability Weights for Each Level of Modified Rankin Scale,” Stroke, 40:3828-33.
Publisher – Google Scholar
[22] Cadilhac, D. A., Carter, R., Thrift, A. G. & Dewey, H. M. (2009). “Recursive Partition Algorithmer R. Estimating the Long-Term Costs of Ischemic and Hemorrhagic Stroke for Australia: New Evidence Derived from the North East Melbourne Stroke Incidence Study (NEMESIS),” Stroke, 40:915-921.
Publisher – Google Scholar
[23] Moroney, J. T., Bagiella, E., Paik, M. C., Sacco, R. L. & Desmond, D.W. (1998). “Risk Factors for Early Recurrence After Ischemic Stroke: The Role of Stroke Syndrome and Subtype,” Stroke, 29:2118-2124.
Publisher – Google Scholar
[24] Kirshner, H. S. (2009). “Differentiating Ischemic Stroke Subtypes: Risk Factors and Secondary Prevention,” Journal of the Neurological Sciences, 279:1-8.
Publisher – Google Scholar
[25] Whisnant, J. P., Wiebers, D. O., O’Fallon, W. M., Sicks, J. D. & Frye, R. L. (1996). “A Population-Based Model of Risk Factors for Ischemic Stroke: Rochester, Minnesota,” Neurology, 47:1420-1428.
Publisher – Google Scholar
[26] Hankey, G. J., Jamrozik, K., Broadhurst, R. J., Forbes, S., Burvill, P. W., Anderson, C. S. & Stewart-Wynne, E. G. (1998). “Long-Term Risk of First Recurrent Stroke in the Perth Community Stroke Study,” Stroke, 29:2491-2500.
Publisher – Google Scholar
[27] Van Walraven, C., Hart, R. G., Singer, D. E., Laupacis, A., Connolly, S., Petersen, P., Koudstaal, P. J., Chang, Y. & Hellemons, B. (2002). “Oral Anticoagulants vs Aspirin in Nonvalvular Atrial Fibrillation: An Individual Patient Meta-Analysis,” JAMA The Journal of the American Medical Association, 288:2441-2448.
Publisher – Google Scholar
[28] Fisher, C. M. (1968). “The Arterial Lesions Underlying Lacunes,” ActaNeuropathol, 12:1-15.
Publisher – Google Scholar
[29] Fine-Edelstein, J. S., Wolf, P. A., O’Leary, D. H., Poehlman, H., Belanger, A. J., Kase, C. S. & D’Agostino, R. B. (1994). “Precursors of Extracranial Carotid Atherosclerosis in the Framingham Study,” Neurology, 44:1046-1050.
Publisher – Google Scholar
[30] Sacco, R. L., Benjamin, E. J., Broderick, J. P., Dyken, M., Easton, J. D., Feinberg, W. M., Goldstein, L. B., Gorelick, P. B., Howard, G., Kittner, S. J., Manolio, T. A., Whisnant, J. P. & Wolf, P. A. (1997). “American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk Factors,” Stroke, 28:1507-1517.
Publisher – Google Scholar
[31] Alter, M., Friday, G., Lai, S. M., O’Connell, J. & Sobel, E. (1994). “Hypertension and Risk of Stroke Recurrence,”Stroke, 25:1605-1610.
Publisher – Google Scholar
[32] Lavados, P. M., Sacks, C., Prina, L., Escobar, A., Tossi, C., Araya, F., Feuerhake, W., Galvez, M., Salinas, R. & Alvarez, G. (2007). “Incidence, Case-Fatality Rate, and Prognosis of Ischaemic Stroke Subtypes in a Predominantly Hispanic-Mestizo Population in Iquique, Chile (PISCIS Project): A Community-Based Incidence Study,” The Lancet Neurology, 6:140-148.
Publisher – Google Scholar
[33] SHEP Cooperative Research Group. (1991). “Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons with Isolated Systolic Hypertension. Final Results of the Systolic Hypertension in the Elderly Program (SHEP),”JAMA The Journal of the American Medical Association, 265:3255-3264.
Publisher – Google Scholar
[34] Friday, G., Alter, M. & Lai, S. M. (2002). “Control of Hypertension and Risk of Stroke Recurrence,” Stroke, 33:2652-2657.
Publisher – Google Scholar
[35] Lakhan, S. E. & Sapko, M. T. (2009). “Blood Pressure Lowering Treatment for Preventing Stroke Recurrence: A Systematic Review and Meta-Analysis,” International Archives of Medicine, 2(1):30.
Publisher – Google Scholar
[36] Arboix, A., García-Eroles, L., Massons, J. & Oliveres, M. (1998). “Predictive Clinical Factors of In-Hospital Mortality in 231 Consecutive Patients with Cardioembolic Cerebral Infarction,” Cerebrovascular Diseases, 8:8— 13.
Publisher – Google Scholar
[37] Hata, J., Tanizaki, Y., Kiyohara, Y., Kato, I., Kubo, M., Tanaka, K., Okubo, K., Nakamura, H., Oishi, Y., Ibayashi, S. & Iida, M. (2005). “Ten Year Recurrence after First Ever Stroke in a Japanese Community: The Hisayama Study,” Journal of Neurology, Neurosurgery & Psychiatry, 76(3):368-72.
Publisher – Google Scholar
[38] Mohan, K. M., Crichton, S. L., Grieve, A. P., Rudd, A. G., Wolfe, C. D. & Heuschmann, P. U. (2009). “Frequency and Predictors for the Risk of Stroke Recurrence up to 10 Years after Stroke: The South London Stroke Register,” Journal of Neurology, Neurosurgery & Psychiatry, 80(9):1012-8.
Publisher – Google Scholar
[39] Béjot, Y., Rouaud, O., Jacquin, A., Osseby, G. V., Durier, J., Manckoundia, P., Pfitzenmeyer, P., Moreau, T. & Giroud M. (2010). “Stroke in the Very Old: Incidence, Risk Factors, Clinical Features, Outcomes and Access to Resources–A 22-Year Population-Based Study,” Cerebrovascular Diseases, 29(2):111-21. Epub 2009 Dec 1.
Publisher – Google Scholar
[40] Hassan, A. E., Zacharatos, H., Suri, M. F. & Qureshi, A. I. (2007). “Drug Evaluation of Clopidogrel in Patients with Ischemic Stroke,” Expert Opinion Pharmacotherapy, 8:2825-2838.
Publisher – Google Scholar
[41] Meyer, D. M., Albright, K. C., Allison, T. A. & Grotta, J. C. (2008). “LOAD: A Pilot Study of the Safety of Loading of Aspirin and Clopidogrel in Acute Ischemic Stroke and Transient Ischemic Attack,” Journal of Stroke & Cerebrovascular Diseases, 17:26-29.
Publisher – Google Scholar
[42] Hermann, A., Dzialowski, I., Koch, R. & Gahn, G. (2009). “Combined Anti-Platelet Therapy with Aspirin and Clopidogrel: Risk Factor for Thrombolysis-Related Intracerebral Hemorrhage in Acute Ischemic Stroke?,” Journal of the Neurological Sciences, 284:155-157.
Publisher – Google Scholar
[43] Calvet, D., Touze, E. & Mas, J. L. (2006). “Adding Aspirin to Clopidogrel in Secondary Prevention of Ischemic Stroke: No Significant Benefits. Results of the Match Study,” Presse Medicale, 35:679-682.
Publisher – Google Scholar
[44] Hachinski, V., Donnan, G. A., Gorelick, P. B., Hacke, W., Cramer, S. C., Kaste, M., Fisher, M., Brainin, M., Buchan, A. M., Lo, E. H., Skolnick, B. E., Furie, K. L., Hankey, G. J., Kivipelto, M., Morris, J., Rothwell, P. M., Sacco, R. L., Smith, S. C. Jr., Wang, Y., Bryer, A., Ford, G. A., Iadecola, C., Martins, S. C., Saver, J., Skvortsova, V., Bayley, M., Bednar, M. M., Duncan, P., Enney, L., Finklestein, S., Jones, T. A., Kalra, L., Kleim, J., Nitkin, R., Teasell, R., Weiller, C., Desai, B., Goldberg, M. P., Heiss, W. D., Saarelma, O., Schwamm, L. H., Shinohara, Y., Trivedi, B., Wahlgren, N., Wong, L. K., Hakim, A., Norrving, B., Prudhomme, S., Bornstein, N. M., Davis, S. M., Goldstein, L. B., Leys, D. & Tuomilehto, J. (2010). “Stroke Synergium. Stroke: Working toward a Prioritized World Agenda,” Cerebrovascular Diseases, 30(2):127-47. Epub 2010 May 24.
Publisher – Google Scholar









