Introduction
The long-term cumulative prevalence of dementia in Parkinson’s disease (PD) has been reported to be as high as 80% (Aarsland et al.,2003), while mild cognitive impairment (MCI) occurs in 20-30% of PD subjects (Caviness et al.,2007; Muslimovic et al.,2005). Recognition of early cognitive impairment in PD is important since it represents a risk factor for developing Parkinson’s disease dementia (PDD) and psychosis. Poor quality of life, increased caregiver burden and a shorter life expectancy have also been associated with cognitive impairment in PD subject (Marras et al., 2008; Forsaa et al., 2010). As a consequence, accurate cognitive assessment and classification are very relevant.
Neuropsychological testing is the current gold standard for assessing cognition, but it is time consuming and many clinicians lack access to such assessments. It has been suggested that an ideal cognitive screening instrument in PD should be brief, evaluate a range of cognitive domains, be simple to administer, sensitive to the initial stage of cognitive impairment and unaffected by motor impairment (Nazem et al., 2009). Few screening instruments have been validated or developed to evaluate global cognition in PD. The Mini-Mental State Examination (MMSE) (Folstein et al., 1975) remains the most commonly used screening instrument for global cognition, even though it has not been specifically validated for use in PD subjects (Litvan et al., 2012). More recently, the Montreal Cognitive Assessment (MoCA) test has been recommended as a better screening tool in PD (Nasreddine et al., 2005; Chou et al., 2010).
Despite the lack of an optimal screening cutoff score for PD-MCI in the MoCA, a score of 26 or less has been proven to provide a sensitivity and negative predictive value above 80%, with a specificity ranging from 52% to 75%. Several studies have consistently shown that the MoCA is a more sensitive instrument for the detection of PD-MCI compared to the MMSE (Zadikoff et al., 2008).
Nevertheless, the overall discriminant validity for the detection of any cognitive disorder in PD is similar for the MoCA test and the MMSE (Hoops et al., 2009)). Most of the previously mentioned data come from studies carried out in developed countries which have a higher mean of formal years of education. Mexico is among the lower performing OECD (Organisation for Economic Co-operation and Development) countries in reading, mathematics and science (http://www.oecd.org/education/eag.htm)
Ethnic differences in MMSE scores have been reported. Mexican Americans were 2.2 times more likely than European Americans to have MMSE scores <24 (Espino et al., 2001). A low MMSE sensitivity and specificity in subjects with a low level of education (<4 years) has also been reported in Mexican population (Ostrosky-Solis et al., 2000). To our knowledge, no published information regarding the MoCA test in Mexican population has been yet published.
The aim of this study is to compare the performance of the MMSE and MoCA test in terms of cognitive impairment classification in PD subjects with a low educational level.
Subjects and Methods
A prospective consecutive sample of subjects with PD fulfilling the criteria for Parkinson’s disease according to the United Kingdom Parkinson’s Disease Society – Brain Bank (Lees et al.,2009) were assessed. Subjects currently receiving anticholinergic drugs or with identified secondary factors associated with cognitive impairment were excluded.
Demographic and clinical data related to PD were obtained. The level of education was defined as complete years of formal schooling received. In Mexico, high school is defined as 12 years of formal education (elementary and secondary school). The Spanish versions of the MoCA test and the MMSE were administered in a systematically counterbalanced fashion to avoid the order effects. Items common to both tests were asked only once. A score of less than 26 for both the MMSE and the MoCA test was used as a cutoff value. For domain analysis, one error in any item was considered as the failure of the whole domain for the study purposes. Trained research staff applied both tests. If educational years were less than twelve, a point was added to total score in MoCA.
A neurologist with expertise in movement disorders performed the clinical evaluation. Disease severity was graded in terms of the Hoehn and Yahr (HY) staging (Hoehn et al., 1967) and motor evaluation was performed using the Short Parkinson’s Evaluation Scale/Scales for Outcomes in Parkinson’s disease (SPES/SCOPA). The SPES/SCOPA is a short scale with good reliability and validity when compared to the UPDRS Part III. Score ranges from 0 to 63 with a higher scores relating to greater motor impairment (Marinus et al., 2004). All subjects were on their “on” state at the time of assessment.
The Institutional Review Board and local ethics committee approved the study. All subjects provided a written informed consent before participating.
Statistical Analysis
Total scores on the MMSE and MoCA test were compared using unpaired t tests. Normality of the distribution was assessed by the Shapiro-Wilk test; when the normality assumption did not hold, non-parametric tests were performed. Comparison of nominal variables was performed using a chi-square test. Agreement between the two different clinical tools to determine the presence or absence of cognitive decline was assessed by the kappa coefficient (≤0=poor, .01—.20=slight, .21—.40=fair, .41—.60=moderate, .61—.80=substantial, and .81—1=almost perfect) [19]. A multiple regression analysis was used to determine factors that impact total score in MoCA and MMSE; independent variables included in the model were age, sex, years of schooling, Hoehn and Yahr stage, use of levodopa, use of dopamine agonist and use of monoamine oxidase inhibitors. Statistical analysis was performed using SPSS Version 16 (SPSS Inc. Chicago, IL, USA).
Results
A total of one hundred twenty eight patients (79 males and 49 females) were included. The main demographic and clinical characteristics of the sample are shown in Table 1. The mean of total years of schooling was 8.7 ± 4.9. Fifty-two patients (40.5%) had completed high school, sixty-six patients (52%) did not complete high school and 10 (8.1%) received no formal education.
Table 1: Clinical and Demographic Characteristics of Subjects

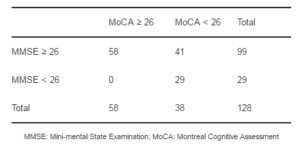
The mean MMSE and MoCA scores of the sample were 27±3.9 and 23.2±5.8, respectively. Clinimetric properties of MMSE and MoCA are shown in Table 2. A greater proportion of patients scored less than 26 in the MoCA test (55%) than in the MMSE (23%). Moreover, 41.4% of the patients classified as normal by the MMSE, scored less than 26 points on the MoCA test. Table 3 shows the data for the paired ratings as a 2×2 contingency table. The kappa coefficient measurement of agreement was fair (k=0.39).
Table 2: Clinimetric Properties of MMSE and MoCA
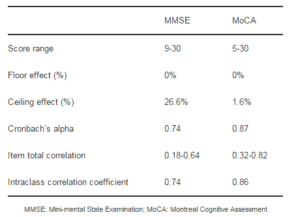
Table 3: Number of Patients with a Score ≥26 and <26 for Each Test
In the MoCA, the domains with the greater percentage of errors include memory, visuospatial and attention. In the MMSE language and attention were the most impaired domains. Table 4 shows the percentages of errors per equivalent domain for both instruments.
Table 4: Percentage of Patients with Error per Domain in MMSE and MoCA

Multiple regression analysis showed that the years of schooling (B=0.45, p>00.1), age (B=-2.27, p=0.03) and HY stage (B=-3.49, p=0.001) were significantly correlated with the total score of the MoCA. For MMSE, only the years of schooling proved to be an independent predictor (B=-2.5, p=0.01).
Discussion
Early detection of cognitive impairment in PD subjects is required in order to implement therapeutic measures to improve the quality of life, increase life expectancy and diminish caregiver burden, as well as the risk of developing dementia (Marras et al., 2008). Therefore, the need exists for a highly sensible screening tool for MCI in PD subjects.
The mean years of education in our sample were lower when compared to other studies where the MoCA and MMSE have been compared [Zadikoff et al., 2008). Nazem et al reported a mean of 15.7 ± 3.6 years of education for their study; a similar study also carried out in the United States reported a mean of 16.1 ± 3.1 years of education (Hoops et al., 2009). This has many implications in regards to the clinimetric and screening properties found for both tests. It has been reported that poorly educated subjects are more likely to show a floor effect on the MMSE (Franco-Marina et al., 2010). On the other hand, MMSE ceiling effects are more frequent in highly educated subjects (van Gorp et al., 1999). We found a higher ceiling effect for MMSE than for MoCA in our population. Previous studies have described the MoCA as less prone to present ceiling effects. Additionally, in Mexican subjects with less than four years of schooling, the specificity of the MMSE for dementia detection has been reported to be of 50% (Ostrosky-Solis et al., 2000).
In this study, a greater proportion of PD subjects had a score below the suggested cut-off value for the assessment of MCI when using the MoCA test. This suggests that the MoCA test is a more useful test for the detection of MCI in Mexican PD subjects.
The recognition of MCI according to the MoCA has been reported to be frequent in PD subjects without evidence of cognitive impairment based on MMSE performance. In our population, 41% of subjects having a “normal” MMSE score were classified as having MCI when the MoCA test was considered; this frequency is similar to what has been previously reported. In a study conducted on two movement disorder centers in the United States, up to 52% of PD patients with a normal MMSE score met the criteria for cognitive impairment on the MoCA test (Nazem et al., 2009). A study in Japanese population reported that two thirds of PD subjects with a normal MMSE score showed cognitive impairment according to the MoCA test (Seki et al., 2012).
The difference of MoCA for the detection of cognitive impairment may be due to the broad range of cognitive domains tested. While the MMSE focuses primarily on memory and language abilities, the MoCA examines domains that have been proven to be particularly impaired in PD subjects. PD-MCI seems to be characterized by frontal-subcortical impairment; with greater deficiency in prefrontal tasks, visuospatial skills and memory. The onset of posterior cortical impairment portrays the transition to P (Williams-Gray et al., 2009). It has been reported that all of the visuospatial assessments on the MoCA test, particularly the cube-copying task, are more sensitive to MCI than the interlocking pentagon task on the MMSE (Alty et al, 2012). In our study, subjects classified as MCI on the MoCA had more mistakes on memory, visuospatial/executive function, and attention. Additionally, the MoCA test is considered to be more difficult than the MMSE (Nazem et al., 2009). In our study the kappa coefficient of agreement was fair, but this should be interpreted cautiously since the same rater applied both tests, and this could contravene the assumption of independence. This bias is expected to inflate the magnitude of kappa.
The primary limitation of the current study is the absence of a comprehensive neuropsychological assessment to provide a criterion standard diagnosis of MCI. This prevents the evaluation of sensitivity, specificity and predictive values of MoCA for our population and impedes the subclassification of PD-MCI. Nevertheless, the evaluation of diagnostic accuracy was not the objective of the present study. A second limitation is the lack of direct comparison between subjects with low- and high-education profile. A final limitation of this study is the lack of a validated screening cutoff score for PD-MCI for both: MoCA and MMSE. On the other hand, a score of 26 or less has been used for most published studies [Marras et al., 2008; Nazem et al., 2009); Hoops et al., 2009; Dalrymple-Alford et al., 2010); this cutoff score has shown to provide great sensitivity, although specificity may be decreased.
Conclusion
This is the first study to analyze the use of MoCA as a screening test for MCI in subjects with PD with a lower education level. The current study found that the MoCA classifies a greater proportion of PD subjects as having cognitive impairment. This suggests the use of MoCA will allow the recognition of more subjects who require further specific neuropsychological testing. As such, the MoCA seems to be of further use as a screening tool than the MMSE for the detection of PD-MCI in poorly educated subjects. Results from our analysis could be directly applied to other samples with a high proportion of poorly educated participants.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A. & Kragh-Sorensen, P. (2003). “Prevalence and Characteristics of Dementia in Parkinson Disease: An 8-Year Prospective Study,” Archives of Neurology, 60 (3):387-392.
Publisher – Google Scholar
Alty, J. E., Smith, S. L. & Jamieson, S. (2012). ‘Screening for Cognitive Impairment in Parkinson’s Disease and Age-Matched Controls Using Mmse and Moca: Which Visuospatial Tests are Most Sensitive?,’ Movement Disorders Journal,27(Suppl 1):32.
Google Scholar
Caviness, J. N., Driver-Dunckley, E., Connor, D. J., Sabbagh, M. N., Hentz, J. G., Noble, B., Evidente, V. G., Shill, H. A. & Adler, C. H. (2007). “Defining Mild Cognitive Impairment in Parkinson’s disease,” Movement Disorders Journal,22(9):1272-1277.
Publisher – Google Scholar
Chou, K. L., Amick, M. M., Brandt, J., Camicioli, R., Frei, K., Gitelman, D., Goldman, J., Growdon, J., Hurtig, H. I., Levin, B., Litvan, I., Marsh, L., Simuni, T., Troster, A. I., Uc, E. Y. & Parkisnon Study Group (2010). “A Recommended Scale for Cognitive Screening in Clinical Trials of Parkinson’s Disease,” Movement Disorders Journal, 25(15):2501-2507.
Publisher – Google Scholar
Dalrymple-Alford, J. C., Macaskill, M. R., Nakas, C. T., Livingston, L., Graham, C., Crucian, G. P., Melzer, T. R., Kirwan, J., Keenan, R., Wells, S., Porter, R. J., Watts, R. & Anderson, T. J. (2010). “The Moca: Well-Suited Screen for Cognitive Impairment in Parkinson Disease,” Neurology, 75(19):1717-1725.
Publisher – Google Scholar
Espino, D. V., Lichtenstein, M. J., Palmer, R. F. & Hazuda, H. P. (2001). “Ethnic Differences in Mini-Mental State Examination (MMSE) Scores: Where You Live Makes a Difference,” Journal of the American Geriatrics Society, 49(5):538-548.
Publisher – Google Scholar
Folstein, M. F., Folstein, S. E. & Mchugh, P. R. (1975). “Mini-Mental State. A Practical Method for Grading the Cognitive State of Patients for the Clinician,” Journal of Psychiatric Research, 12(3):189-198.
Publisher – Google Scholar
Forsaa, E. B., Larsen, J. P., Wentzel-Larsen, T. & Alves, G. (2010). “What Predicts Mortality in Parkinson Disease?: A Prospective Population-Based Long-Term Study,” Neurology, 75(10):1270-1276.
Publisher – Google Scholar
Franco-Marina, F., Garcia-Gonzalez, J. J., Wagner-Echeagaray, F., Gallo, J., Ugalde, O., Sánchez-García, S., Espinel-Bermudez, C., Juárez-Cedillo, T., Rodriguez, M. & García-Pe-a, C. (2010). “The Mini-Mental State Examination Revisited: Ceiling and Floor Effects after Score Adjustment for Educational Level in an Aging Mexican Population,”International Psychogeriatrics, 22(1):72-81.
Publisher – Google Scholar
Hoehn, M. M. & Yahr, M. D. (1967). “Parkinsonism: Onset, Progression and Mortality,” Neurology, 17(5):427-442.
Publisher – Google Scholar
Hoops, S., Nazem, S., Siderowf, A. D., Duda, J. E., Xie, S. X., Stern, M. B. & Weintraub, D. (2009). “Validity of the Moca and Mmse in the Detection of Mci and Dementia in Parkinson Disease,” Neurology, 73(21):1738-1745.
Publisher – Google Scholar
Lees, A. J., Hardy, J. & Revesz, T. (2009). “Parkinson’s Disease,” The Lancet, 373(9680):2055-2066.
Publisher
Litvan, I., Goldman, J. G., Troster, A. I., Shmand, B. A., Weintraub, D., Petersen, R. C., Mollenhauer, B., Adler, C. H., Marder, K., Williams-Gray, C. H., Aarsland, D., Kulisevsky, J., Rodriguez-Oroz, M. C., Burn, D. J., Barker, R. A. & Emre, M. (2012). “Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines,” Movement Disorders Journal, 27(3):349-356.
Publisher – Google Scholar
Marinus, J., Visser, M., Stiggelbout, A. M., Rabey, J. M., Martínez-Martin, P., Bonuccelli, U., Kraus, P. H. & Van Hilten, J. J. (2004). “A Short Scale for the Assessment of Motor Impairments and Disabilities in Parkinson’s Disease: The SPES/SCOPA,” Journal of Neurology, Neurosurgery and Psychiatry, 75(3):388-395.
Publisher – Google Scholar
Marras, C., Mcdermott, M. P., Rochon, P. A., Tanner, C. M., Naglie, G., Lang, A. E. & Parkinson Study Group (2008). “Predictors of Deterioration in Health-Related Quality of Life in Parkinson’s Disease: Results from the DATATOP Trial,” Movement Disorders Journal, 23(5):653-659.
Publisher – Google Scholar
Muslimovic, D., Post, B., Speelman, J. D. & Schmand, B. (2005). “Cognitive Profile of Patients with Newly Diagnosed Parkinson Disease,” Neurology, 65(8):1239-1245.
Publisher – Google Scholar
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L. & Chertkow, H. (2005). “The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment,” Journal of the American Geriatrics Society, 53(4):695-699.
Publisher – Google Scholar
Nazem, S., Siderowf, A. D., Duda, J. E., Havet, T., Colcher, A., Horn, S. S., Moberg, P. J., Wilkinson, J. R., Hurtig, H. I., Stern, M. B. & Weintraub, D. (2009). “Montreal Cognitive Assessment Performance in Patients with Parkinson’s Disease with “Normal” Global Cognition according to Mini-Mental State Examination Score,” Journal of the American Geriatrics Society, 57(2):304-308.
Publisher – Google Scholar
Ostrosky-Solis, F., Lopez-Arango, G. & Ardila, A. (2000). “Sensitivity and Specificity of the Mini-Mental State Examination in a Spanish-Speaking Population,” Applied Neuropsychology, 7(1): 25-31.
Publisher – Google Scholar
Seki, M., Kobari, M., Mihara, B., Isozumi, K., Ohta, K., Muramatsu, K. & Shirai, T. (2012). ‘Screening Utility of Montreal Cognitive Assessment (Moca) For Detecting Cognitive Impairment in Patients with Parkinson’s Disease,’ Movement Disorders Journal, 27(Suppl 1):102.
Google Scholar
Sim, J. & Wright, C. C. (2005). “The Kappa Statistic in Reliability Studies: Use, Interpretation, and Sample Size Requirements,” Physical Therapy, 85(3):257-268.
Publisher – Google Scholar
Van Gorp, W. G., Marcotte, T. D., Sultzer, D., Hinkin, C., Mahler, M. & Cummings, J. L. (1999). “Screening for Dementia: Comparison of Three Commonly Used Instruments,” Journal of Clinical and Experimental Neuropsychology,21(1):29-38.
Publisher – Google Scholar
Williams-Gray, C. H., Evans, J. R., Goris, A., Foltyne, T., Ban, M., Robbins, T. W., Brayne, C., Kolachana, B. S., Weinberger, D. R., Sawcer, S. J. & Barker, R. A. (2009). “The Distinct Cognitive Syndromes of Parkinson’s Disease: 5 Year Follow-Up of the Campaign Cohort,” Brain, 132(Pt 11):2958-2969.
Publisher – Google Scholar
Zadikoff, C., Fox, S. H., Tang-Wai, D. F., Thomsen, T., De Bie, R. M., Miyasaki, J., Duff-Canning, S., Lang, A. E. & Marras, C. (2008). “A Comparison of the Mini Mental State Exam to the Montreal Cognitive Assessment in Identifying Cognitive Deficits in Parkinson’s Disease,” Movement Disorders Journal, 23(2):297-299.
Publisher – Google Scholar






