Introduction
Zirconium oxide has many applications in the nuclear and chemical industries. It is the starting material for fabrication of ceramic and metallic fuels proposed to be used widely in nuclear industry. Since zircaloy is resistant to embrittlement and has very low neutron absorption cross section, it is used as a cladding material in all Pressurized Heavy Water Reactors (PHWR). Zirconium oxide is the starting material for the production of zicaloy. It is used in low amounts in the fabrication of plate type of fuels such as Al-U alloy to gain the desired hardness for the fuel. In view of this, purity requirements of ZrO2 are similar to those of nuclear fuel materials, specially from neutronics point of view. Besides nuclear industry, zirconium oxide is used as a catalyst as well as a catalyst support. Having good chemical stability and excellent electric conduction, ZrO2-based ceramics have been widely applied in manufacturing piezoelectricity components, capacitors, solid electrolyte fuel battery, etc. Because the impurities in high purity ZrO2 powder strongly influence its properties it is of great importance to determine these trace elements. Trace elemental assay of ZrO2 thus, assumes great significance from the viewpoint of quality assurance.
Atomic Emission Spectrometry (AES), which is a simultaneously multi-elemental analytical technique with the advantages of acceptable precision and sensitivity, is regularly used for the trace metal assay of nuclear fuels and associated materials [Satyanarayana et al (2010), Rajeswari et al (2002), Sengupta et al (2010, 2011), Bangia et al (1988), Porwal et al (1990), Page et al (1977, 1983) and Purohit et al (2000)].However, analytical technique based on AES suffers from the spectral interferences. In particular, nuclear materials containing uranium, plutonium, thorium, zirconium due to their emission rich spectra necessitates a development of suitable separation procedure for removal of major matrix without any loss of analytes prior to their determination. Presence of emission rich matrix like U, Pu, Zr can lead to erronius determination of trace constituents due to spectral interference [Sengupta et al (2013, 2014)]. A number of separation procedures have been reported to achieve trace metal assay of nuclear materials either by chemically [Satyanarayana et al (2010), Rajeswari et al (2002), Sengupta et al (2010, 2011), Bangia et al (1988), Argekar et al (2002), Mahan et al (2000), Malhotra et al (1999), Marin et al (1996) and Huff et al (1989)] or physically [Bangia et al (1988), Porwal et al (1990), Page et al (1977, 1983 and Dalvi et al (1978)]. In dc arc carrier distillation technique suitable carrier is used to sweep out the trace metallic constituents into the arc leaving the matrix into the graphite electrode. Due to the less sample handling the probability of chance contamination is also found to be less.
In the present case an attempt was made to develop a methodology for determination of trace metallic constituents in ZrO2 matrix by carrier distillation dc arc spectroscopic method where a mixture of AgCl and SrF2 was used as a carrier to sweep out the trace constituents into the arc and also maintain the arc stability and temperature. The method developed appears to be promising for the determination of 19 impurities with RSD ~15%.
Experimental
Instrumentation
A 0.75M, Jarrel Ash Direct Reading Spectrometer, fitted with 48 analytical channels, forms the basic operating system. The spectrometer is equipped with d.c. arc and ICP as the sources of spectral excitation. A PDP 11/23 computer along with LA-120 DEC writer is used as a process controller for data acquisition and processing. A RSX-11M basic system supports the software used in the analytical procedure.
Electrodes
Lower electrode (anode): Standard carrier distillation type electrode, viz. ASTM designation E-130-66 type S-2 used on type S-1 pedestal. Upper electrode (cathode): Preformed pointed electrode, ASTM designation E-130-66 type C-I.
Preparation of standard and samples
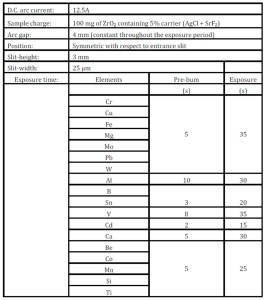
The analytes of interest in the present studies were: Co, Cr, Mg, B, Be, Cd, Sn, Mn, Cu, Ni, V, Ca, Pb, Fe, Ti, Al, Mo, W and Si. A master standard was prepared by mixing known amounts of the oxides for most of these elements with high purity pre-analyzed zirconium oxide. A standard charge of 100 mg thus contained 5% AgCl — SrF2 mixture (AgCl: SrF2 = 4: 1), graded proportion of the master standard containing trace amounts of the metallic elements of interest and pure ZrO2. Three ZrO2 samples were prepared synthetically with varying concentrations of trace metals covering the analytical range for precision studies.
Procedure
The choice and proportion of the carrier combination were first optimized by studying the volatilization/excitation characteristics of all the metallic elements. The time-gating facility associated with the direct reading emission spectrometer was used in these studies. The analyte line intensity for an intermediate concentration standard was monitored for one second at each of the sixty three positions available for study of the arc during the exposure period. Other experimental parameters such as arc current, radial and axial position of the arc plasma etc. were then optimized for gaining high analytical signal for all the elements using the standard prepared with optimized carrier combination. The signal integration time for each of the analytes was also optimized from the study of their volatilization/excitation behavior and finally the analytes were grouped into seven sets of pre-burn and signal integration periods as shown in more detail along with other optimized parameters in Table 1. The d.c. arc analytical response being, in general, a nonlinear one, detailed calibration process was adopted here. The calibration curve for each analytical channel was obtained by using the data based on the replicate observations for each of the entire series of standards.
Table 1: Optimization of instrumental and experimental parameters for the analysis
Results and discussion
A systematic study was carried out to develop a methodology for the determination of trace metallic constituents in ZrO2 matrix. As neutrons are the primary particles causing nuclear fission, their economy is of utmost importance. Elements like B, Cd and some rare earth elements like Eu, Sm, Gd, Dy have very large neutron absorption cross section and their presence results in the loss of neutrons and hence need to be monitored and controlled at ultra trace level. Low melting elements cause liquid metal embrittlement altering the fuel structure. Refractory elements like W, Mo etc. cause creep resistance resulting in clad damage. The presence of light elements results the change in density required for the successful operation of the nuclear reactors at desired burn — up. Fe, Ni, Cr are generally monitored to check the process pick up and the condition of the process equipments. The presence of Fe and Ni at high concentration leads to the sintering problem. The overall impurity content deteriorates the chemical and metallurgical properties of the substance. On view of this it is required to have a critical chemical and physical quality control of the nuclear fuels and associated materials.
Since Zr is multi-elemental being rich in emission spectra is expected to interfere during the determination of other analytes at the trace levels. In the present method, a carrier mixture is chosen to sweep out the trace constituents into the arc leaving major matrix into the graphite electrode. The elements form refractory compound at arcing conditioned cannot be determined at very low concentration. The carrier mixture also makes the arc stable and maintains constant arc temperature. So a careful choice of carrier is required to achieve the required detection limits and determination range with acceptable precession. In the present case, 5% carrier consisting of a mixture of AgCl and SrF2 (4:1) was used for physical separation of the trace constituents from the zirconium matrix. The sample is placed inside the cup like anode which is on the graphite pedestal while the cathode is pointed to minimize the arc wandering.
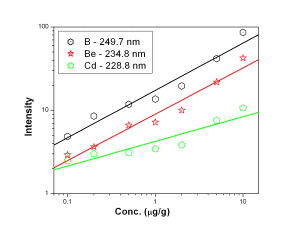
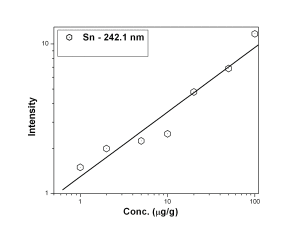
Fig. 1 represents the calibration curves of Co, Cr and Mg, It was observed that though the background intensity of Co 228.6 nm line is more than that of 242.4 nm line, the sensitivity of the former line is an order of magnitude better. 425.4 nm emission line of Cr was found to have slightly better analytical performance with respect to sensitivity and detection limit in comparison with 357.8 nm line of Cr. Mg 280.2 nm line was found to have lower background as well as better sensitivity than 282.5 nm analytical channel. The calibration curves for B- 249.7 nm, Be — 234.8 nm and Cd — 228.8 nm lines were shown in Fig. 2. The calibration curves for B, Be and Cd were obtained in the concentration range of 0.1 µg/g — 10 µg/g while that of Sn 242.1 nm line [Fig. 3] was 1 µg/g — 100 µg/g.
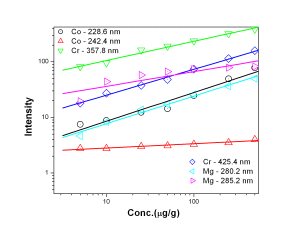
Figure 1: Calibration curves for Co, Cr and Mg
Figure 2: Calibration curves for B, Be and Cd
Figure 3: Calibration curve for Sn
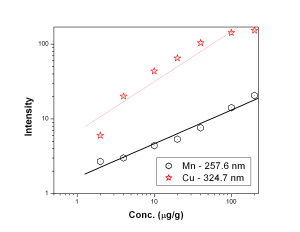
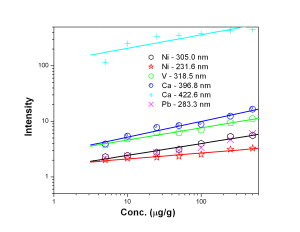
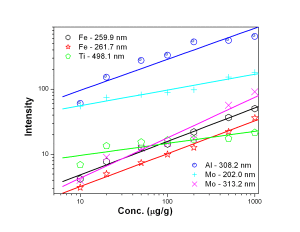
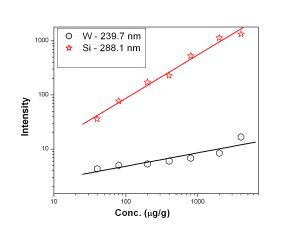
Fig. 4 represent the calibration curves for Mn 257.6 nm and Cu 324.7 nm showing a saturation behavior above 100 µg/g for Mn 257.6 nm line. Calibration curves for Ni, V, Ca and Pb represented by Fig. 5 suggested that the calibration curves for the above mentioned elements were established in the concentration range of 5 — 500 µg/g. Ni 305.0 nm line was found to have double sensitivity than the 231.6 nm line inspite of similar detection limits. Both the analytical lines of Ca i.e. 396.8 nm and 422.6 nm were found to have similar analytical performance. Fe 261.7 nm line was found to have lower detection limit than Fe 259.9 nm line though both of the lines were having similar sensitivity whereas Mo 313.2 nm line is of better analytical performance than 202.0 nm line [Fig. 6]. Fig. 7 represents the calibration curves for W and Si, which suggests that above 2000 µg/g, there is saturation of signal for 288.1 nm Si line due to self-absorption.
Figure 4: Calibration curves for Mn and Cu
Figure 5: Calibration curves for Ni, V, Ca and Pb
Figure 6: Calibration curves for Fe, Ti, Al and Mo
Figure 7: Calibration curves for W and Si
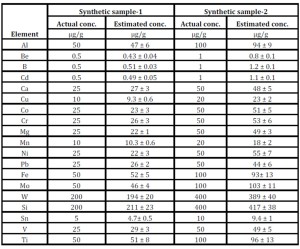
The method was validated using two synthetic samples and it was found to be suitable for the determination of Co, Cr, Mg, B, Be, Cd, Sn, Mn, Cu, Ni, V, Ca, Pb, Fe, Ti, Al, Mo, W and Si in Zr matrix without any chemical separation with an RSD of ~15%. Table -2 represents the analytical results of the synthetic samples.
Table 2: Determination of trace metallic analytes in Synthetic samples
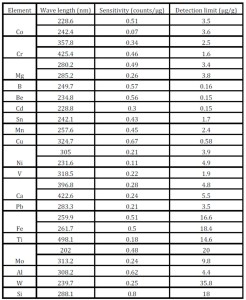
The sensitivity and the detection limits of all the above mentioned analytes were calculated and summarized in Table – 3. The sensitivity was calculated on the basis of the slope of the calibration curves while the detection limit is the concentration corresponding to the intensity of + 3σ, where is the average of the blank intensity value and σ is the standard deviation of the blank measurements. The detection limits were found to be in the range of 0.15 µg/g to 36 µg/g.
Table 3: Determination of the sensitivity and the detection limits of the analytes
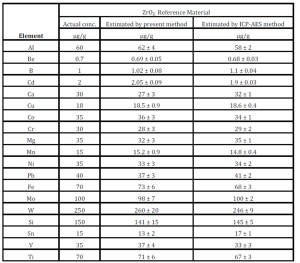
A comparative study was carried out to validate the present methodology with the routinely employed ICP-AES based method using reference ZrO2 material, certified by Round-Robin experiments (inter laboratory comparison experiment) carried out in different laboratories of Department of Atomic Energy, India. The routinely adopted method for trace metal assay in a Zr matrix is chemical separation of Zr (quantitatively) from the analytes using 30% TBP—dodecane (five contacts) from an aqueous solution of ~4 M HNO3, followed by feeding of the aqueous phase into the plasma for analysis[Sengupta et al (2013)]. The above-mentioned routine procedure was adopted and the data were compared with the newly developed methods. The analytical results were summarized in Table 4. The results were found to be satisfactory.
Table 4: Validation of the present method using ZrO2 reference material and its comparison with routinely employed method
Conclusion
Zirconium being one of the key elements of nuclear fuel and associated materials, a careful control of its chemical purity is required for the desired performance in the reactors. The present study deals with the development of an analytical method based on dc arc carrier distillation atomic emission spectrometry for simultaneous determination of 18 trace metallic impurities in zirconium matrix. A mixture of AgCl and SrF2 was used as a carrier for the physical separation of trace constituents from the matrix into the arc to minimize the spectral interference of zirconium on other analytes even at trace level. The experimental parameters were optimized and it was found that the method is promising for the determination of Co, Cr, Mg, B, Be, Cd, Sn, Mn, Cu, Ni, V, Ca, Pb, Fe, Ti, Al, Mo, W and Si in Zr matrix at the trace level with an overall precession of 15%. The methodology was validated using certified reference material using routinely employed methodology.
References
1. Argekar, A.A., Kulkarni, M.J., Mathur, J.N. and Page, A.G. (2002) Chemical separation and ICP-AES determination of 22 metallic elements in U and Pu matrices using cyanex-923 extractant and studies on stripping of U and Pu, Talanta 56 (4), 591-601
Publisher – Google Scholar
2. Bangia T.G., Dhawale B.A., Adya V.C. and Sastry M.D. (1988) ICP-AES and dc arc AES determination of Sc, Y and lanthanides in nuclear grade graphite, Fresenius J Anal Chem, 332, 802-804
Publisher – Google Scholar
3. Dalvi A.G.I., Deodhar C.S., Seshagiri T.K., Khalap M.S. and Joshi B.D., (1978) Determination of refractory elements in U3O8 by carrier distillation emission spectrography, Talanta, 25, 665-668.
Publisher – Google Scholar
4. Huff E.A. and Bowers D.L. (1989) The Determination of Impurities in Plutonium Metal by Anion Exchange and ICP/AES, Appl. Spectroscopy, 43(2), 223-226.
Publisher – Google Scholar
5. Mahan, C., Bonchin, S., Figg, D., Gcrth, D. and Collier, C. (2000) Chromatographic extraction of plutonium and inorganic impurity analysis using ICP-MS and ICP-AES, Journal of Anal Atom Spec, 15 (8), 929-935.
Publisher – Google Scholar
6. Malhotra, R.K. and Satyanarayana, K. (1999) Estimation of trace impurities in reactor-grade uranium by ICP-AES, Talanta 50 (3), 601-608.
Publisher – Google Scholar
7. Marin, S., Cornejo, S., Jara, C. and Duran, N. (1996) Determination of trace level impurities in uranium compounds by ICP-AES after organic extraction, Fresenius’ Journal of Analytical Chemistry 355 (5-6), 680-683.
8. Page A.G., Godbole S.V., Deshkar S., Babu Y. and Joshi B.D. (1977) Spectrographic determination of metallic impurities in PuO2, Fresenius J Anal Chem, 287, 304-309.
Publisher – Google Scholar
9. Page A. G., Madraswala K. H., Godbole S. V., Kulkarni Madhuri J., Mallapurkar Vanita S., and B. D. Joshi (1983),Carrier Distillation-ICAP Approach for Trace Metal Assay of U3O8 Powder, Fresenius Z Anal Chem 315, 38-41.
Publisher – Google Scholar
10. Porwal N. K., Argekar A. A., Pnrohit P. J., Page A. G. and M. D. Sastry, (1990) Trace metal assay of thorium oxide by atomic emission spectrometry, Fresenius J Anal Chem, 338, 255-258.
Publisher – Google Scholar
11. Purohit, P.J., Goyal, N., Thulasidas, S.K., Page, A.G., Sastry, M.D. (2000) Electrothermal vaporization – inductively coupled plasma-atomic emission spectrometry for trace metal determination in uranium and thorium compounds without prior matrix separation, Spectrochimica Acta B, 55 (8), 1257-1270
Publisher – Google Scholar
12. Rajeswari, B., Dhawale, B.A., Bangia, T.R., Mathur, J.N. and Page, A.G., (2002) Role of Cyanex-272 as an extractant for uranium in the determination of rare earths by ICP-AES, J Radioanal Nucl Chem 254 (3), 479-483.
Publisher – Google Scholar
13. Satyanarayana, K. and Durani, S. (2010) Separation and inductively coupled plasma optical emission spectrometric (ICP-OES) determination of trace impurities in nuclear grade uranium oxide, J Radioanal. Nucl. Chem. 285 (3), 659-665.
Publisher – Google Scholar
14. Sengupta Arijit, Adya V. C., Acharya R., Mohapatra P. K. and Manchanda V. K., (2010) Characterization of purified 241Am for common impurities by instrumental neutron activation analysis, J Radioanal Nucl Chem, 287(1), 281-285
15. Sengupta A., Kulkarni M. J. and Godbole S. V. (2011) Analytical application of DHOA for the determination of trace metallic constituents in U based fuel materials by ICP-AES, J Radioanal Nucl Chem, 289(3), 961-965
Publisher – Google Scholar
16. Sengupta A., Rajeswari B., Kadam R. M. and Kshirsagar R. J. (2011) Characterization of serpentine: a potential nuclear shielding material, J Radioanal Nucl Chem, 292 (2), 903-908
17. Sengupta Arijit, Adya V.C., Seshagiri T.K. and S.V. Godbole (2013) Exploration of CCD based ICP-AES for Studying Spectral Interferences of Uranium on Other Analytes, Atomic Spectroscopy, 34(2)53 -58.
18. Sengupta Arijit and Adya V.C. (2013) Determination of Common Analytes at Trace Levels in Zr Matrix by ICP-AES Without chemical/Physical Separation, Atomic Spectroscopy, 34(6), 207-215.
19. Sengupta Arijit, Adya V. C. and Godbole S. V. (2014) Spectral interference study of uranium on other analytes by using CCD based ICP-AES, J Radioanal Nucl Chem. DOI 10.1007/s10967-013-2520-0