Introduction
The diagnostic efficacy of FNAC in identifying recurrences and metastasis in mesenchymal lesions is undebatable. The use of FNAC in primary diagnosis of mesenchymal lesions, especially of the malignant category, is still open to a lot of scepticism among pathologists and clinicians. As the management of most of these lesions involves definitive and sometimes radical surgery, the necessity arises for determining the category of the neoplasms as basically benign or malignant. If not the specific diagnosis, it becomes imperative for the surgeon to be aware of the primary diagnosis of mesenchymal lesion prior to surgery as basically benign or malignant, as it helps in subsequent patient management. Sensing this necessity, the present study is undertaken to determine the usefulness, limitations and diagnostic accuracy of FNAC in primary evaluation of mesenchymal lesions. FNAC as a pre-operative diagnostic technique is definitely an attractive alternative to open biopsy, since it is relatively inexpensive, non-invasive and does not require hospitilization, besides providing the clinician and patient with rapid preliminary diagnosis, allowing them to consider management decisions during the first hospital visit, without losing out on the precious time factor in diagnosis.
Materials and Methods
In this study, 502 patients who underwent FNAC for mesenchymal lesions during a period 5 years from 2007 to 2011 were reviewed. The cytology smears were obtained by standard FNAC procedure using 22-24 gauge needle, under aseptic precautions. The aspirates thus obtained were immediately fixed in 95% ethanol for subsequent staining by Papanicolaou method.
Amongst 502 cases , 256 cases of typical lipomas, 77 cases of fibrolipomas and 42 cases of cytological smears with inadequate material were excluded from the study. Of the remaining 127 cases, histological correlation was available in 113 cases and only these cases were taken up, for calculation of various statistical indices.
FNAC diagnosis were stratified into three general categories as benign, malignant and suspicious of malignancy.The last subset included those cases where the differential diagnosis included at least one malignant lesion, but specific categorization as benign/malignant was not possible. Since FNAC is a screening test with a relatively high sensitivity for the diagnosis of malignancy, the cases under the category of ‘suspicious of malignancy’ were considered in the category of malignant lesions for statistical analysis. After reviewing the cytology smears and histopathology sections independently, cytohistological correlation was done.
Special Stains: Per-iodic acid Schiff (PAS) and Phosphotungstic acid hematoxylin (PTAH) were done in cases with histopathologial diagnosis of Ewing`s sarcoma and Rhabdomyosarcoma.
IHC study was undertaken in 3 cases for confirmation of diagnosis to facilitate appropriate patient management.
Observations
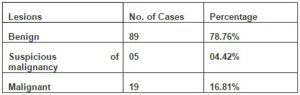
Out of the 113 cases in which histopathological correlation was available, 89 (78.76%) cases were diagnosed as benign, 5(4.42%) as suspicious of malignancy and 19(16.81%) as malignant lesions (Table 1).The benign cases which correlated on histology were neurofibroma (38 cases), benign fibrous histiocytoma (8 cases), schwannoma (8 cases), benign spindle cell tumor (6 cases), neurofibrolipoma (5 cases), fibromatosis (5 cases), pleomorphic lipoma(3 cases), fibroma (3 cases), nodular fasciitis (2 cases), benign smooth muscle tumor (2 cases of leiomyoma), giant cell tumor of tendon sheath (2 cases), benign vascular tumor (2 cases) and xanthofibroma (1 case). The malignant cases included 4 cases of liposarcoma, 3 cases of malignant fibrous histiocytoma, 2 cases each of alveolar rhabdomyosarcoma, spindle cell sarcoma, pleomorphic sarcoma and myxoid sarcoma and 1 case each of osteosarcoma, small round cell sarcoma and epithelioid/ polygonal cell sarcoma.
Table 1: Distribution of Lesions (Cytological)
Of the 89 cytologically benign cases, 85 (95.51%) correlated on histology. 4 (4.49%) cases which showed cytohistologic discordance included one case each of MPNST, synovial sarcoma, well- differentiated liposarcoma and low –grade fibrosarcoma. 18 of the 19 cases with cytodiagnosis of malignancy were subsequently confirmed as malignant on hisopathology.
One false positive case of benign fibrous histiocytoma was cytologically diagnosed as DFSP (Table 2 & 3).
Table 2: Cytohistological Correlation
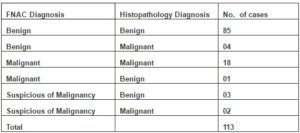
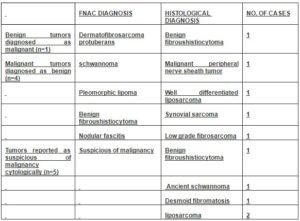
Table 3: Summary of Cases with Cytohistologic Discordance
As FNAC is used as a screening test with a relatively high sensitivity for malignancy, cases diagnosed as ‘suspicious of malignancy ‘were included under the malignant lesions for statistical analysis.
Of the 5 cases diagnosed cytologically as ‘suspicious of malignancy ‘, 2 proved to be malignant (liposarcoma) and 3 benign (one case each of benign fibrous histiocytoma, ancient schwannoma and desmoid fibromatosis on histopathology).
Discussion
Neurofibroma, Benign fibrous histiocytoma, and Schwannoma were the most common benign tumors in this study similar to the study by Gonzalez-Campora R et al in1992. Definite cytodiagnosis and subtyping was possible in well-differentiated malignant lesions with distinct cytological features. In this category of well-differentiated malignancies, liposarcoma was the most common entity. The other lesions in this category comprised of Malignant fibrous histiocytoma, Alveolar rhabdomyosarcoma and Osteogenic sarcoma.
Malignant lesions in which cytological features did not permit definite subtyping were grouped under broader categories as ‘Spindle cell sarcomas ‘(Fig 1), Small round cell sarcomas (Fig 2), Epithelioid / polygonal cell sarcomas, Pleomorphic cell sarcomas (Fig 3) and Myxoid sarcomas (Fig 4).
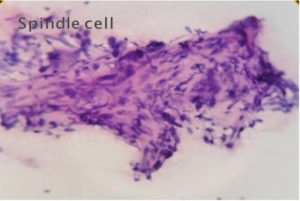
Figure 1-Spindle Cell Sarcoma (H & E, 40)
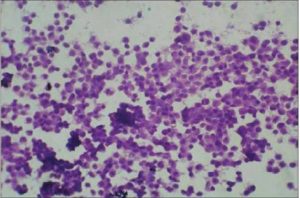
Figure 2-Round Cell Sarcoma (H & E, 40 X)
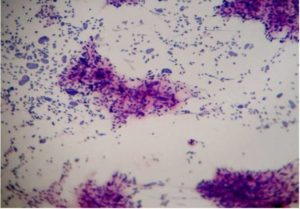
Figure 3-Pleomorphic Sarcoma (H&E, 40 X)
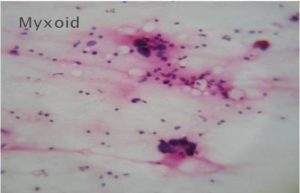
Figure 4-Myxoid Sarcoma (H & E, 40 X)
The subsequent histoplathological examination and special stain (PAS) confirmed cytologically diagnosed Small round cell tumors as Ewing`s sarcoma/PNET .The Spindle cell tumors were confirmed as fibrosarcomas on histopathology. Epitheloid /polygonal cell sarcoma was confirmed as Granular cell tumor on histopathology. The Pleomorphic cell sarcomas were confirmed on histopathology as Pleomorphic rhabdomyosarcoma or Pleomorphic liposarcoma. The Myxoid sarcomas were confirmed as Myxoid liposarcoma or Fibromyxoid sarcoma.
IHC study of three cases confirmed the diagnosis of Malignant fibrous histiocytoma, Synovial sarcoma and Low- grade fibrosarcoma.
FNAC has several advantages over traditional open incisional biopsy for diagnosis and management of malignant neoplasms including little or no risk of tumor cell contamination of the needle track, less risk of morbidity and mortality, ease of performance and rapid turnaround time (Maitra A et al in 2000, Gonzalez-Campora R in 2000). FNA is less expensive, less invasive, can sample a lesion more extensively, and provides quicker results compared with needle core biopsy ( Vincent Y Ng et al in 2010).
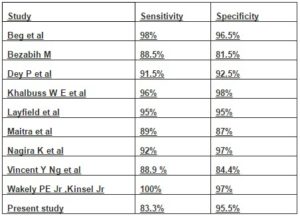
However, FNAC has certain limitations in the diagnosis of some reactive and benign connective tissue lesions with treacherous pleomorphism and ironically, in some sarcomas with monotonous appearance (Hadju S I and Melamed M R in 1984). The sensitivityand specificity of FNAC in correctly classifying primary mesenchymal lesions as malignant were 83% and 95% respectively in the present study, comparing favourably with the observations in other studies (Table 4).
Table 4: Comparative Statistical Analysis of Various Studies
The role of FNAC in differentiating a benign soft tissue lesion from a sarcoma is commendable (Kulkarni D R et al in 2002 , Rekhi B et al in 2007), an opinion which is in concordance with our observation . According to Wakely PE Jr, Kneidsl JS in 2000, FNA cytopathology was capable of specifically subtyping a large percentage of primary and metastatic soft tissue tumors if cellular material either in the form of a cell block or flow cytometry is obtained in addition to cell smears. Dey P et al in 2004 found that FNAC was very useful in distinguishing benign from malignant soft tissue tumors. However, it was not so effective in exact categorization of tumors. In our study, specific subtyping of malignant lesions on cytology posed the greatest challenge. The difficulties in interpretation were probably due to overlap in the cytologic features of these entities. Well-differentiated malignant lesions with distinct cytological features could be diagnosed and specifically subtyped with ease. One of the liposarcomas in the present study was located in the breast, an unusual site.
Bezabih et aI in 2001 categorized malignant lesions in which definite subtyping was not possible on cytology under 5 broader subgroups as, Small round cell sarcomas, Spindle cell sarcomas, Epithelioid/polygonal cell sarcomas, Pleomorphic cell sarcomas and Myxoid sarcomas according to predominant cytomorphologic features. Similar categorization was done in present study to facilitate patient management.
In our study, cytological smears from` round cell sarcomas ‘were highly cellular and were composed of discohesive tumor cells and occasional small cellular clusters, whereas, hypercellularity was a dominant feature in `spindle cell sarcoma’ group .The tumor cells tended to be discohesive and individually dispersed with a few scattered variably sized tumor cell clusters. In contrast to round cell sarcomas, cytological atypia was observed more often in spindle cell sarcomas. Pleomorphism was marked in the Pleomorphic cell sarcomas whereas myxoid sarcomas showed focal myxoid areas amidst tumor cells.
The four false negatives encountered in the study comprised one case each of MPNST, Well-differentiated Liposarcoma, Synovial sarcoma and Low- grade fibrosarcoma. The probable reasons being:
1) Low –grade MPNST may show spindle cells with bland wavy nuclei, thus mimicking a schwannoma as in our case.
2) Well-differentiated liposarcoma may be misdiagnosed as pleomorphic lipoma due to the inability to demonstrate lipoblasts with certainity on cytological smears as in our case and the presence of floret cells in both.(Fig 4 and 5).
3) Synovial sarcoma may be diagnosed as Benign fibrous histiocytoma due to extreme uniformity of cells, lack of nuclear pleomorphism , ovoid to slightly round tumor cells with scant tapering cytoplasm and absence of epithelial cells as in the present case.
4) Low-grade fibrosarcoma may be diagnosed as nodular fasciitis due to paucity of mitotic figures and pleomorphic nuclear characters in nodular fasciitis which may mimic Low- grade fibrosarcoma (Ackerman M in 2005, Sharon W. Weiss et al in 2008) as in the present case.
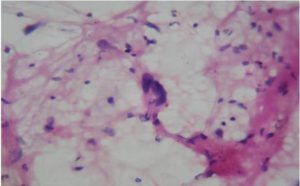
Figure 5-Pleomorphic Lipoma with Floret Cells (H & E, 40 X)
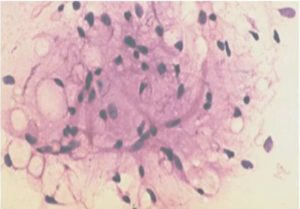
Figure 6- Well Differentiated Liposarcoma Showing Atypical Round to Spindly Cells with Hyperchromatic Nuclei Admixed with Lipoblasts (H & E, 40 X)
Of the 5 cases reported as ‘suspicious of malignancy’, 3 were spindle cell lesions which were confirmed on histopathology as one case each of BFH ,Ancient Schwanomma and Desmoid fibromatosis. On cytologic review, the probable diagnostic pitfalls in these cases were:
- High cellularity with occasional nuclear atypia in BFH.
- Prescence of large atypical cells with occasional atypical mitosis in Ancient schwannoma.
- High cellularity with strands and clusters of cells with moderate anisokaryosis in Desmoid fibromatosis.
The other two cases reported under the ‘suspicious of malignancy’ category were subsequently diagnosed as Well-differentiated liposarcoma on histopathology.
The only false positive under the malignant category in the study was, a case of BFH diagnosed cytologically as DFSP, since they may have cytological similarities like high cellularity, presence of nuclear atypia and histiocytes.
In conclusion, FNAC is a valuable tool in the diagnosis of soft tissue lesions as it does not present major complications and permits a swift preliminary diagnosis in a large number of cases besides being a simple, inexpensive and rapid diagnostic modality. Given the high rates of accuracy, sensitivity and specificity that it offers, coupled with these additional advantages and minimal risks, FNAC represents a viable alternative to open biopsy for the primary diagnosis of soft tissue lesions at initial presentation as it facilitates early decisions and appropriate patient management.
(adsbygoogle = window.adsbygoogle || []).push({});
References
Ackerman, M. (2005). Soft Tissue. In Orell, S. R., Sterret, G. F. & Whitaker, D. editors. ‘Fine Needle Aspiration Cytology,’ 5th Ed. New Delhi: Churchill Livingstone, 387-411.
Google Scholar
Beg, S., Vasenwala, S. M., Haider, N., Ahmad, S. S. et al (2012). “A Comparison of Cytological and Histopathological Findings and Role of Immunostains in the Dagnosis of Soft Tissue Tumors,” J Cytol. 29:125-30.
Publisher – Google Scholar
Bezabih, M. (2001). “Cytological Diagnosis of Soft Tissue Tumors,” Cytopathology. 12(3):177-83.
Publisher – Google Scholar – British Library Direct
Dey, P., Mallik, M. K., Gupta, S. K. & Varishta, R. K. (2004). “Role of Fine Needle Aspiration Cytology in the Diagnosis of Soft Tissue Tumors and Tumor Like Lesions,” Cytopathology, 15:32-37.
Publisher – Google Scholar – British Library Direct
Gonzalez-Campora, R. (2000). “Fine Needle Aspiration Cytology of Soft Tissue Tumors,” Acta Cytol, 44:337-44.
Publisher – Google Scholar
Gonzalez–Campora, R., Munoz–Arias, G., Otal-Salaverri, C., Jara–Heras, M., Gracia-Alvarez, E. & Gomez-Pascual, A. (1992). “Fine Needle Aspiration Cytology of Primary Soft Tissue Tumors. Morphologic Analysis of the Most Frequent Types,” Acta Cytol. 36(6):905-17.
Publisher – Google Scholar – British Library Direct
Hajdu, S. I. & Melamed, M. R. (1984). “Limitations of Aspiration Cytology in the Diagnosis of Primary Neoplasms,” Acta Cytol, 28:337-45.
Publisher – Google Scholar
Khalbuss, W. E., Teot, L. A. & Monaco, S. E. (2010). “Diagnostic Accuracy and Limitations of the Fine Needle Aspiration Cytology of Bone and Soft Tissue Lesions: A Review of 1114 Cases with Cytological Histological Correlation,” Cancer cytopathol, 118(1):24-32.
Publisher
Kulkarni, D. R., Kokandakar, H. R., Kumbhakarna, N. R. & Bhople, K. S. (2002). “Fine Needle Aspiration Cytology of Soft Tissue Tumors in Correlation with Histopathology,” Indian J Pathol Microbiol, 45(1):45-8.
Publisher
Layfield, L. J., Anders, K. H., Glasgow, B. J. & Mira, J. M. (1986). “Fine-needle Aspiration of Primary Soft-tissue Tumors,” Arch Pathol Lab Med, 110:420-4.
Publisher
Maitra, A., Ashfaq, R., Saboorian, M. H., Linderberg, G. & Gokaslan, S. T. (2000). “The Role of Fine –Needle Aspiration Biopsy in the Primary Diagnosis of Mesenchymal Lesions: A Community Hospital –Based Experience,” Cancer (Cancer Cytopathol) 90:178-85.
Publisher – Google Scholar – British Library Direct
Nagira, K., Yamamoto, T., Akisue, T., Marui, T., Hitora, T., Nakatani, T., Kurosaka, M. & Ohbayashi, C. (2002). “Reliability of Fine Needle Aspiration Biopsy in the Intial Diagnosis of Soft Tissue Lesions,” Diagn Cytopathol, 27(6):354-61.
Publisher – Google Scholar – British Library Direct
Ng, V. Y., Thomas, K., Crist, M., Wakely, P. E. & Mayerson, J. (2010). “Fine Needle Aspiration for Clinical Triage of Extremity Soft Tissue Masses,” Clin.OrthoRelatRes. 468(4):1120-1128.
Publisher – Google Scholar
Rekhi, B., Gorad, B. D., Kakade, A. C. & Chinoy, R. F. (2007). “Scope of FNAC in the Diagnosis of Soft Tissue Tumors –A Study from a Tertiary Cancer Referral Center in India,” Cytojournal, 4-20.
Publisher – Google Scholar
Wakely, P. E. & Kinsel, J. S. (2000). “Soft Tissue Aspiration Cytopathology: Diagnostic Accuracy and Limitations,”Cancer. 90(5):292-8.
Publisher – Google Scholar – British Library Direct
Weiss, S. W. & Goldblum, J. R. (2008). Enzinger and Weiss’s soft tissue tumors, 5th Ed.
Publisher












