Experimental
Chemical preparation
The title compound was prepared according to the following chemical reaction:
3 C10H17N + SnCl4 + H2O + 3HCl —> (C10H18N)3[SnCl6]Cl-H2O
by the slow evaporation of an aqueous solution of adamantan-1-amine, tin(IV) chloride dihydrate and hydrochloride acid in 3 : 1 : 3 molar ratio. Colourless prismatic crystals were obtained after one week (yield 65 %). The crystals are stable for months under normal conditions of temperature and humidity. Anal. Calc: C, 42.77; H, 6.41; N, 4.99; Found: C, 42.56; H, 6.57; N, 5.23 %.
Investigation techniques
The characterization of this coordination compound was carried out using X-ray diffraction, solid state NMR and IR spectroscopy.
X-ray single crystal structural analysis
The intensity data were measured at 100 K on a Bruker AXS SMART APEX CCD diffractometer using graphite-monochromated MoKï¡ radiation with the omega scan technique. The absorption corrections were carried out using the Apex2 software [5]. The structure was solved by direct methods using SHELXS and refined by full matrix least squares against F2 with all reflections using SHELXL [6] and SHELXLE [7]. The H-atoms of the water molecules were located from different Fourier maps, while the H-atoms of the ammonium groups were placed in geometrically calculated positions. The drawings were made with Diamond [8]. Crystal data and experimental parameters used for the intensity data collection are summarized in Table 1. The low fraction of observed reflexions is due to the low-quality crystal.
Physical measurements
The NMR spectra were recorded on a solid-state high-resolution Bruker DSX-300 spectrometer operating at 75.49 MHz for 13C and 30.30 MHz for 15N. The 119Sn NMR spectrum was recorded on a Bruker DRX500 AVANCE spectrometer operating at 186.5 MHz with a classical 4 mm probehead allowing spinning rates up to 10 kHz. 13C, 15N and 119Sn NMR chemical shifts are given relative to tetramethylsilane, nitromethane and Me4Sn, respectively (precision 0.5 ppm). The spectra were recorded by the use of cross-polarization (CP) from protons (contact time 5 ms) and MAS. The delay between two successive scans was optimized to ensure a full relaxation of the proton. The IR spectrum was recorded at r. t with a “Perkin-Elmer FTIR” spectrophotometer 1000.
Results and Discussion
X-ray diffraction study
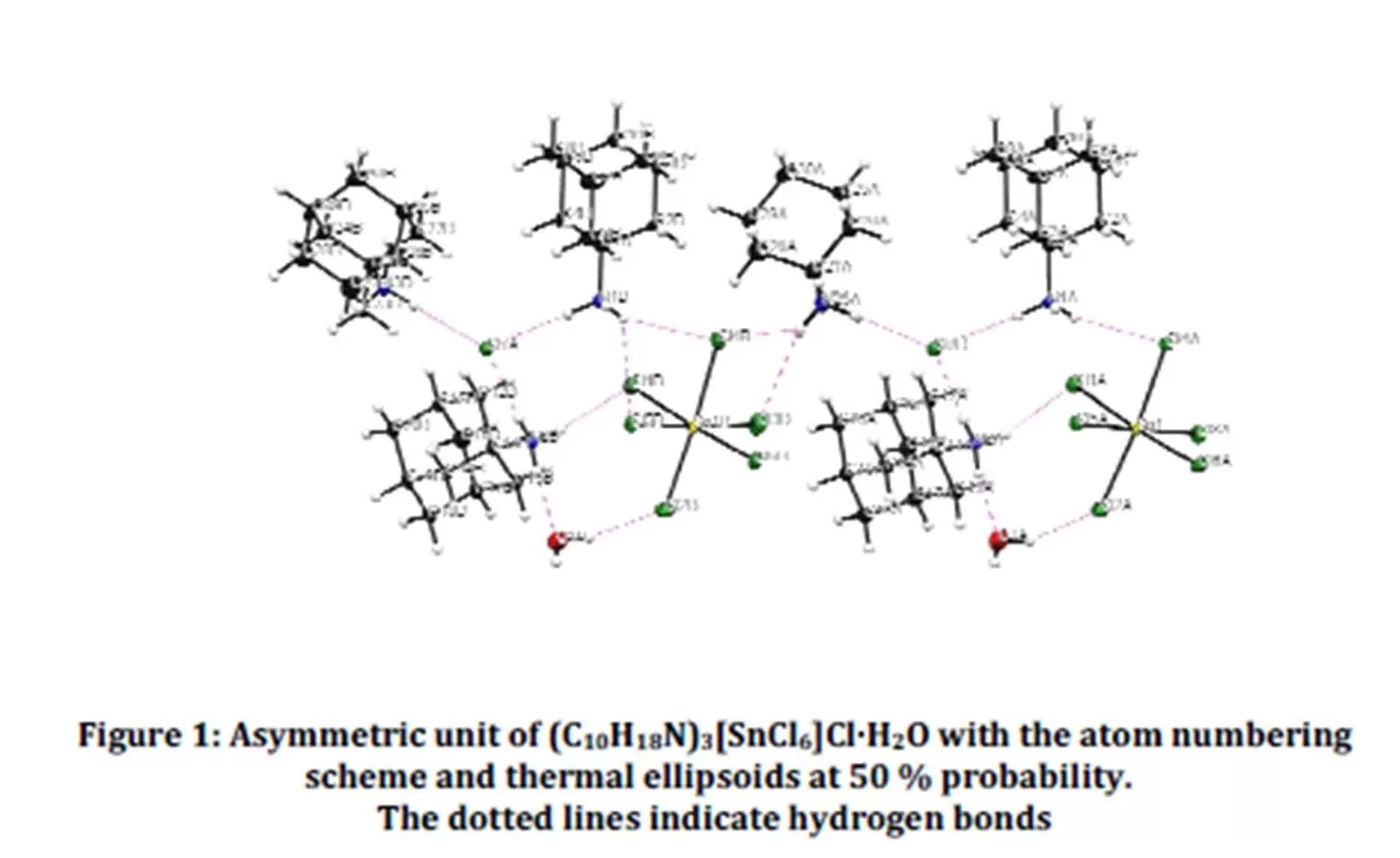
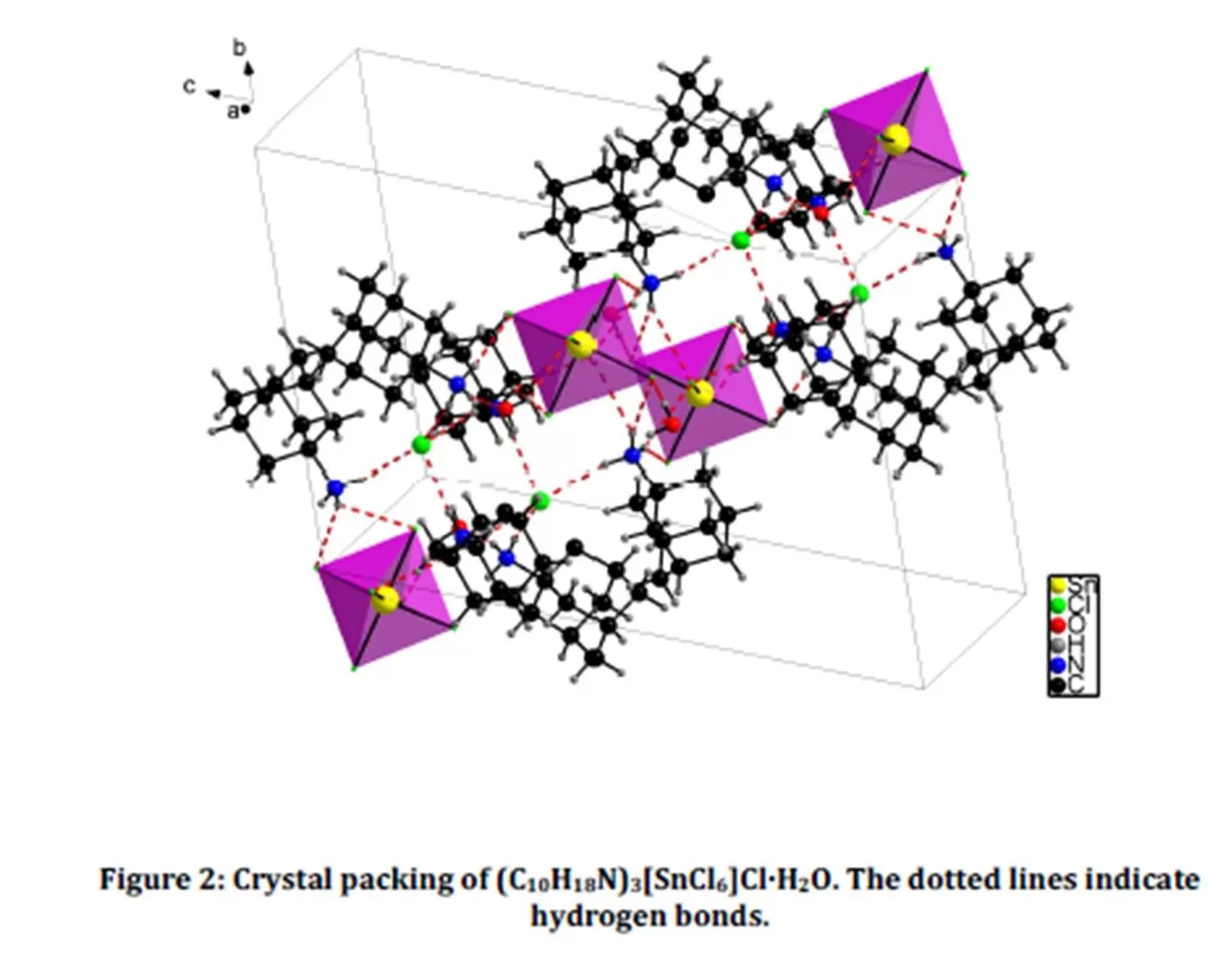
The asymmetric unit of the title compound (Fig. 1) contains six independent adamantan-1-aminium cations, two chloride anions, two Hexachlorostannate (IV) ions and two water molecules. The Sn atom is six-fold coordinated by chloride ions, forming an octahedral arrangement. Fig. 2 shows that the atomic arrangement of the title hybrid compound can be described as inorganic SnCl62- units isolated from each other by the organic cations, the chloride anions and the co-crystallized water molecules. Multiple hydrogen bonds connect the different entities of the compound to form an infinite three-dimensional network. Fig.2 shows that the structure cohesion is ensured by four types of hydrogen bonds, viz. cation-hexachlorotin anion, cation-chloride anion, cation-water and water-hexachlorotin anion. Atoms H3A3, H1B3 H3A1 and H3B2 of the ammonium groups form bifurcated hydrogen bonds to the acceptor atoms
Cl6B, Cl3B, Cl4B, Cl3B, Cl4B, Cl5B, Cl6A and Cl5A (Table 2). The H…Cl distances of these hydrogen bonds, varying between 2.57 and 2.92 Šare longer than the ones of simple hydrogen bonds, ranging from 2.27 and 2.56 Å.
Previous studies have shown that, in the [SnCl6]2- anion, the Sn-Cl bond lengths and Cl-Sn-Cl bond angles are not equal, but vary with the environment around the Cl atom [9]. In this complex, the Sn-Cl bond lengths vary between 2.3791(14) and 2.4694(14) Šand the Cl-Sn-Cl bond angles range from 88.08(5) to 93.65(5)°, with an average value of 89.97° (Table 3). Owing to these differences in the geometric parameters, the [SnCl6]2- anion has a slightly distorted octahedral stereochemistry [10].
The examination of the geometric features of the organic entity shows that the organic molecule exhibits a regular spatial configuration with C-C and C-N distances and C-C-C and C-C-N angles quite similar to those found in other adamantan-1-aminium compounds [11].
NMR spectroscopy
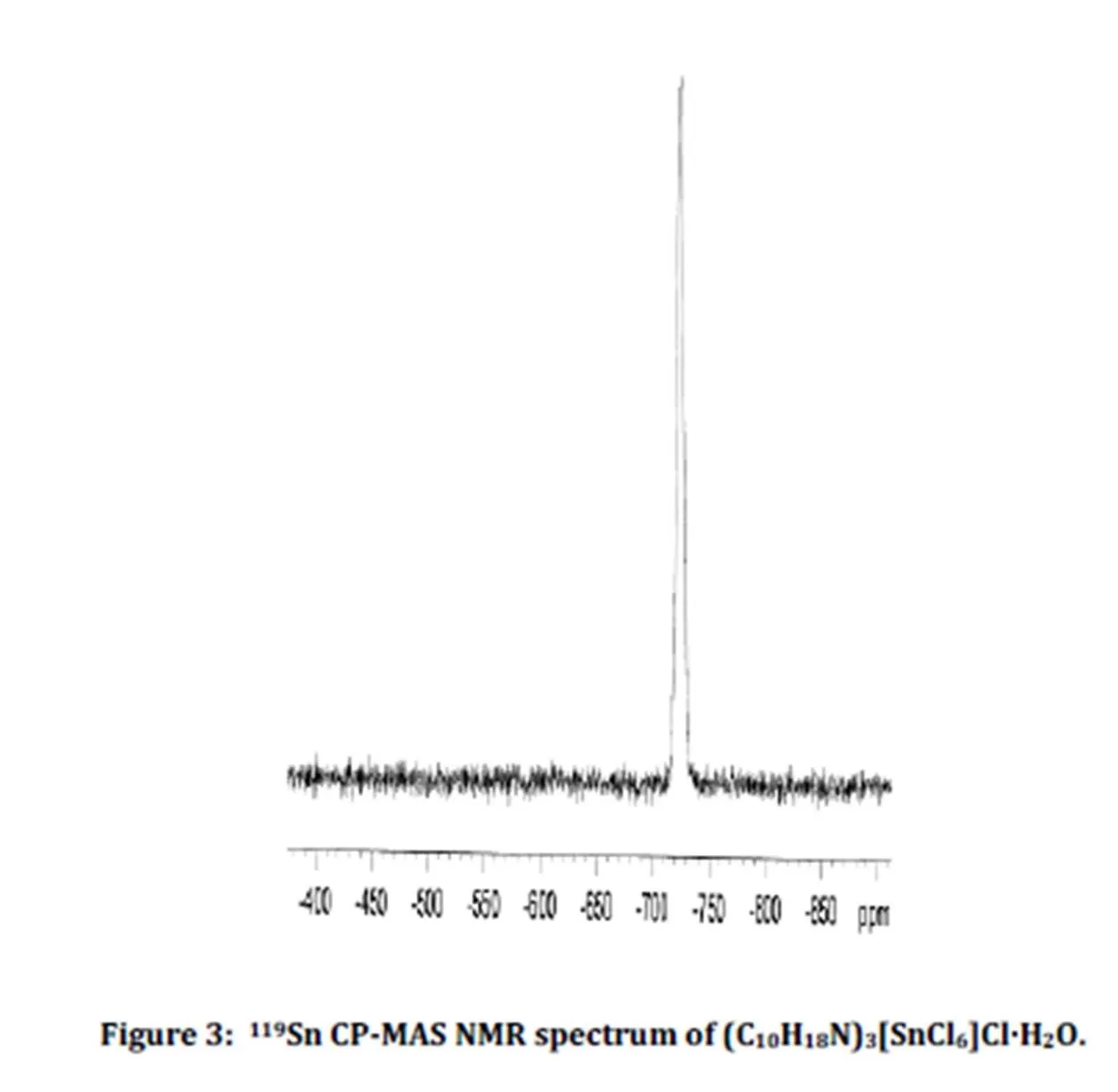
The 119Sn CP-MAS NMR spectrum of crystalline is shown in Fig. 3. It exhibits a single resonance peak at -724 ppm relatively large due to the coupling of the tin (IV) cation with the chlorine atoms. This chemical shift value agrees with those corresponding to the hexachlorostannate (IV) compounds [12]. The single observed peak is in agreement with the presence of only one tin site.
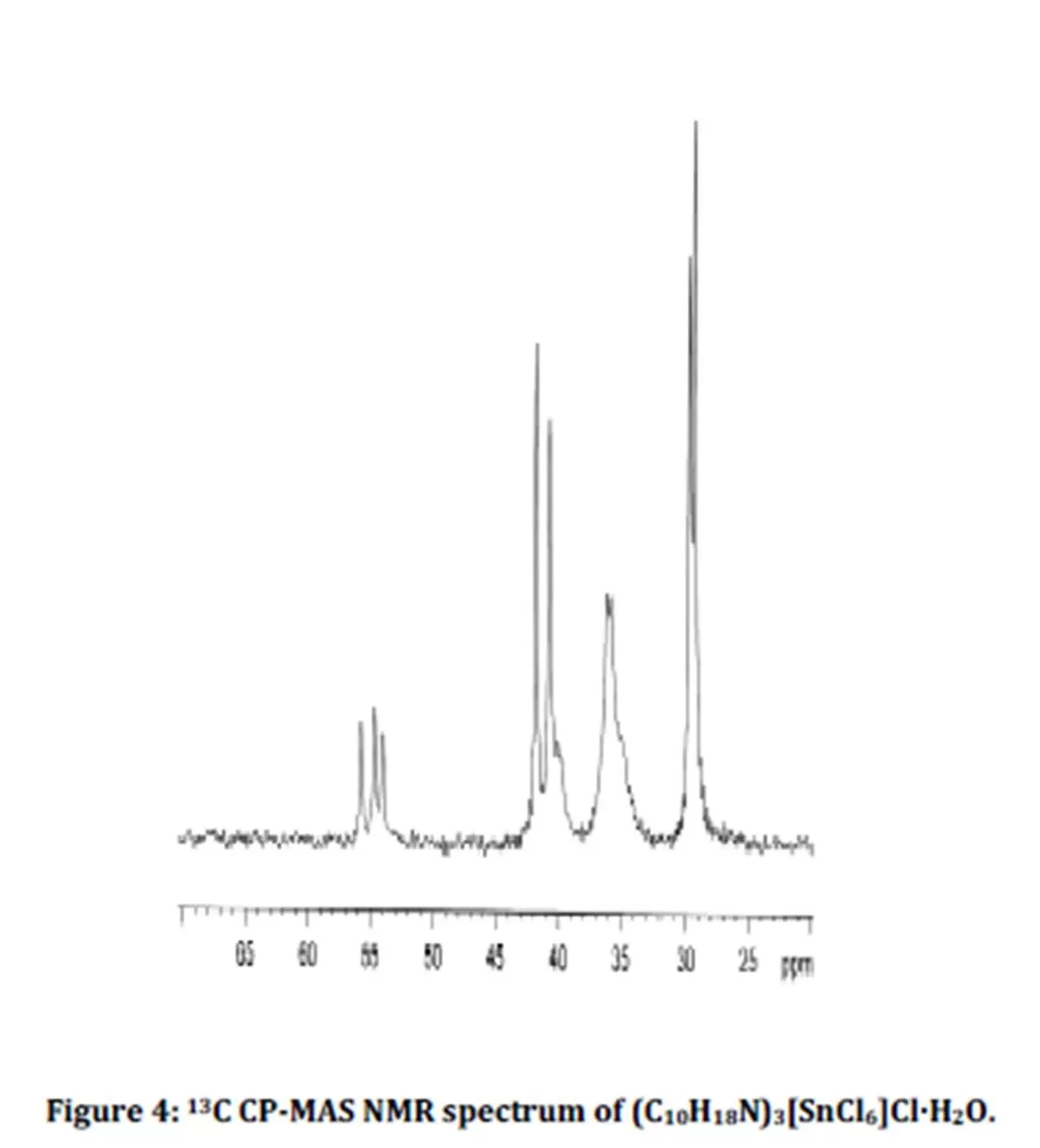
The 13C CP-MAS NMR spectrum of the title compound is shown on Fig. 4. A key point of this spectrum is the presence of three resonances, at 54.1, 55.1 and 55.9 ppm, for the most deshielded signal, corresponding to the carbon atom directly bonded to the ammonium group. These chemical shift values agree with those of literature [13]. These three resonances also confirm the presence of three organic moieties in the asymmetric unit of the compound, in agreement with the X-ray diffraction data.
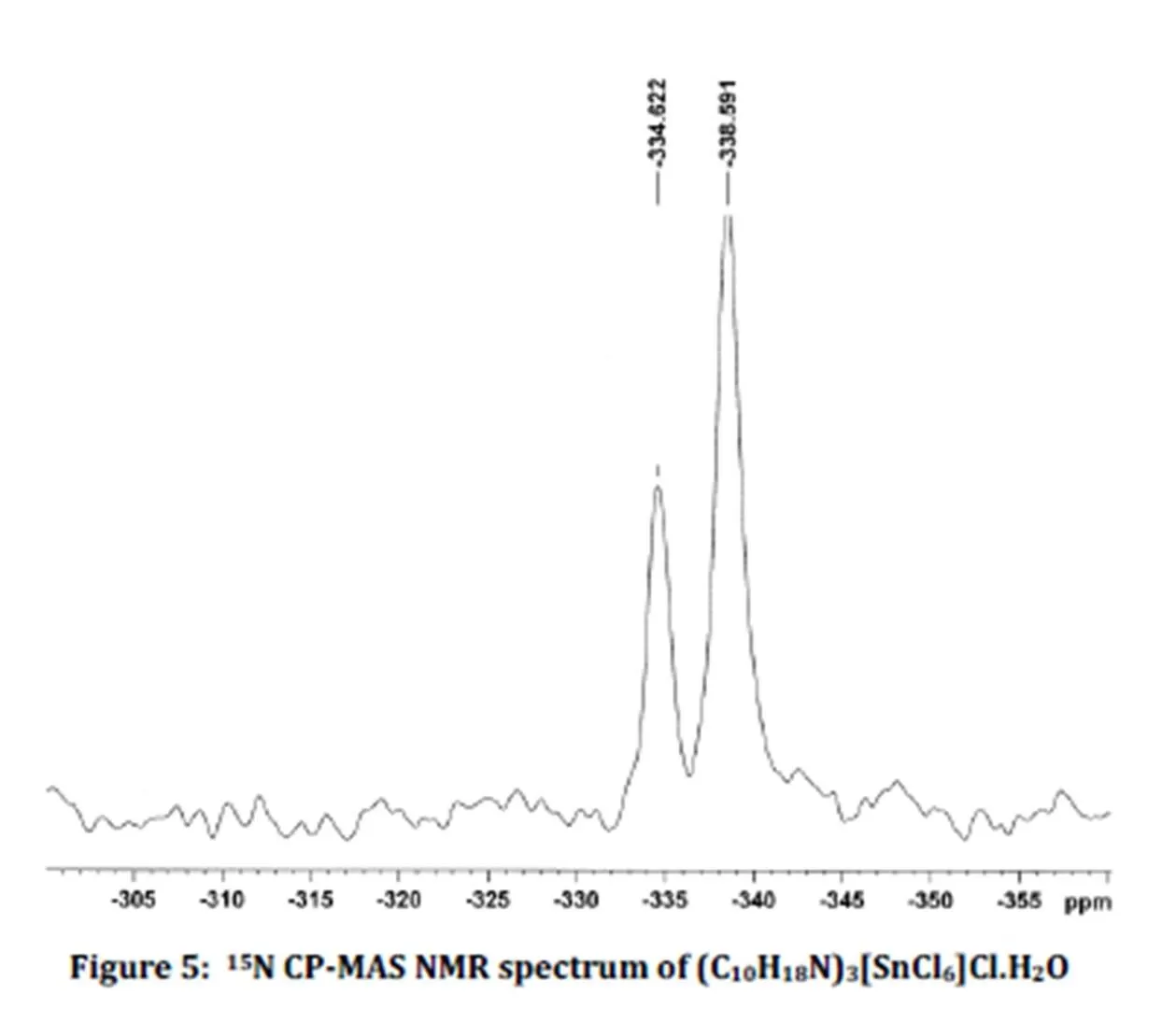
Fig. 5 shows the 15N CP-MAS NMR spectrum of the title compound. This spectrum exhibits two resonance peaks at -338.6 and -334.6 ppm, with 1: 2 relative intensities. The X-ray structure shows that there are three inequivalent nitrogen atoms in the unit cell which is in agreement with the NMR results if one assumes that two inequivalent nitrogen atoms resonate at quite the same value. These chemical shift values agree with those of the nitrogen atom of R-NH3+groups in organic compounds [14].
IR spectroscopy
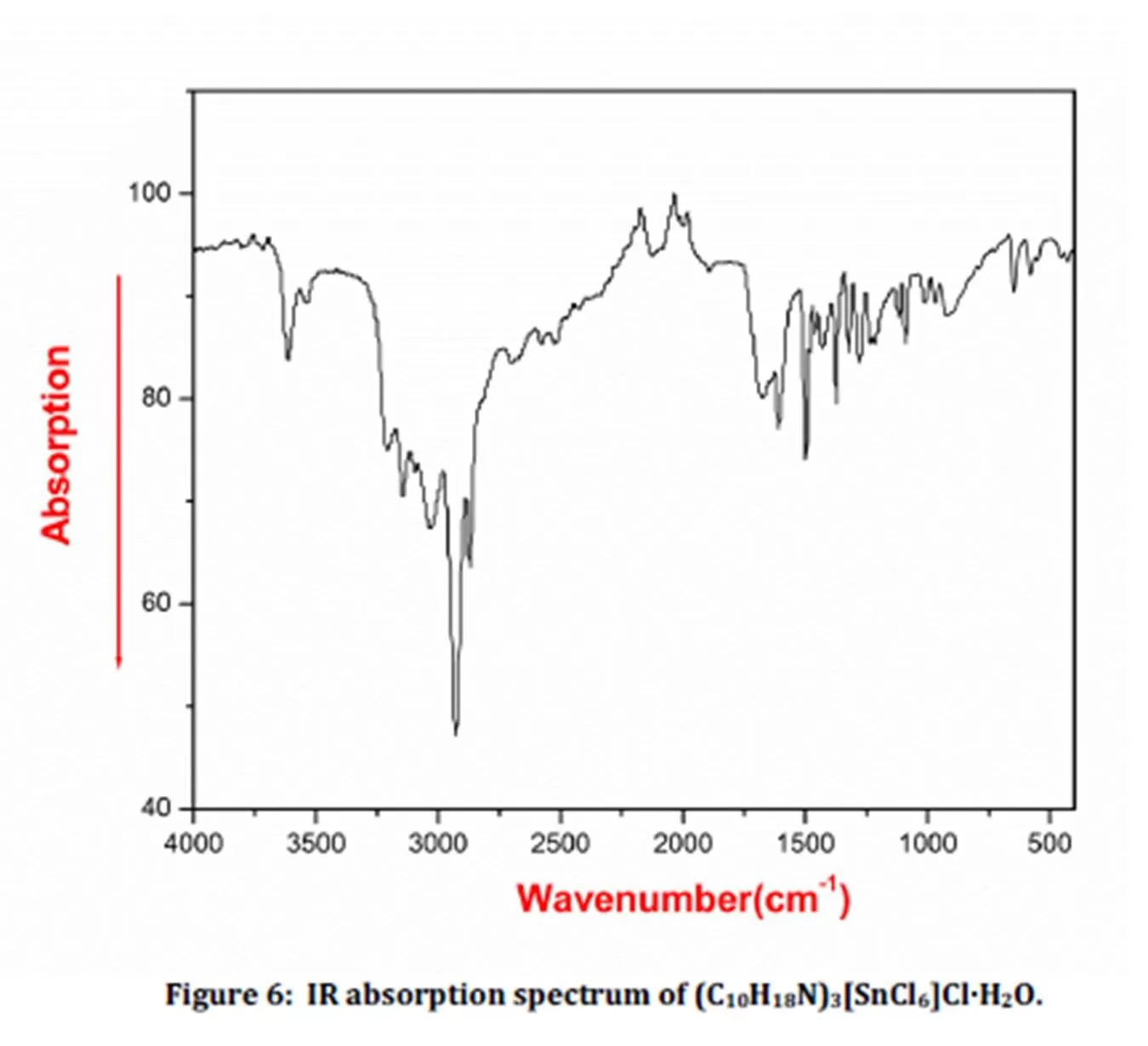
The IR spectrum of crystalline (C10H18N)3[SnCl6]Cl-H2O is shown in Fig. 6. The vibrational modes of this compound were elicited by the comparison of the IR spectra of similar complexes [15, 16]. The broad bands between 3600 and 2500 cm-1 correspond to the stretching vibrations of ï®(C-H), ï®(N-H) and ï®(O-H) groups [17]. The bands in the 1650-1100 cm-1 region can be attributed to the bending vibrations of the ï¤(N-H) and ï¤(O-H) groups and to the stretching and bonding modes ï®(C-C), ï®(C-N) and ï¤(C-H) [18, 19]. The bands between 1000 and 600 cm-1 are assigned to the out of plane bending modes γ(C-H), γ(C-C) and γ(N-H) [20].
Conclusion
A new Sn(IV) complex, (C10H18N)3[SnCl6]Cl-H2O, was synthesized in an aqueous medium and characterized by various physico-chemical methods. On the structural level, the metal centers have 6-coordinated octahedral geometry. Crystal packing is stabilized by intermolecular N-H…Cl, N-H…O and O-H…Cl hydrogen bonds that connect the different entities into a three dimensional lattice. 119Sn, 13C and 15N CP-MAS NMR spectra are in agreement with the X-ray structure.
Supplementary Data
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC 983168. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK: fax: (+44) 01223-336-033; e-mail: deposit@ccdc.cam.ac.
Acknowledgments
We would like to thank the Tunisian Secretariat of State for Scientific Research and Technology for its financial support. Thanks to Professor Matthias Zeller of Youngstown State University (USA) for his fruitful collaboration. The diffractometer was funded by NSF grant 0087210, by Ohio Board of Regents grant CAP-491, and by YSU.
References
1. Aruta, C., Licci, F., Zappettini, A., Bolzoni, F., Rastelli, F., Ferro, P. and Besagni, T. (2005) “Growth and optical, magnetic and transport properties of (C4H9NH3)2MCl4 organic-inorganic hybrid films (M = Cu, Sn),” Applied Physics A, 81(5) 963-968.
Publisher – Google Scholar
2. Knutson, J. L. and Martin, J. D. (2005) “Tuning the Band Gap in Hybrid Tin Iodide Perovskite Semiconductors Using Structural Templating,” Inorgic Chemistry, 44 (13) 4699-4705.
Publisher – Google Scholar
3. Mitzi, D. B., Dimitrakopoulos, C. D. and Kosbar, L. L. (2001) ” Structurally Tailored Organic-Inorganic Perovskites: Optical Properties and Solution-Processed Channel Materials for Thin-Film Transistors,” Chemistry of Materials, 13 (10) 3728-3740.
Publisher – Google Scholar
4. Kagan, C. R., Mitzi, D. B. and Dimitrakopoulos, C. D. (1999) “Organic-Inorganic Hybrid Materials as Semiconducting Channels in Thin-Film Field-Effect Transistors,” Science, 286 (5941) 945-947.
Publisher – Google Scholar
5. Apex2 v2011.2-0, Bruker Advanced X-ray Solutions, Bruker AXS Inc., Madison,Wisconsin, USA.
6. Sheldrick, G.M. (2008) “A short history of SHELX,” Acta Crystallographica, A64112-122.
7. Hübschle, C.B. Sheldrick, G.M. and Dittrich, B. (2011) “ShelXle: a Qt graphical user interface for SHELXL,” Journal Applied Crystallography, 44 (6) 1281-1284.
Publisher – Google Scholar
8. K. Brandenburg, Diamond Version 2.0 Impact GbR, Bonn, Germany, 1998.
9. Rademeyer, M. Lemmerer, A. and Billing, D. G. (2007) “Bis(4-aminopyridinium) hexachlorido-stannate(IV) and bis(ptoluidinium) hexachloridostannate(IV),” Acta Crystallographica, C63 m289-m292.
Publisher – Google Scholar
10. Billing, D. G., Lemmerer, A. and Rademeyer, M. (2007) “Bis(1-phenylethylammonium) hexachloridostannate(IV) and bis(2-phenylethylammonium) hexachloridostannate(IV),”Acta Crystallographica, C63 m101-m104.
Publisher – Google Scholar
11. Mrad, M.L., Zeller, M., Hernandez, K.J. Zraigui, M. and Ben Nasr, C. (2012) “Bis(adamantan-1-aminium) hydrogen phosphate fumaric acid sesquisolvate,” Acta Crystallographica, E68: o2531- o2532.
Publisher – Google Scholar
12. Katayama, H. Kamigaito, M. and Sawamoto, M. (1998) “In-Situ Direct Analysis of the Growing Species by 119 Sn NMR Spectroscopy: Living Cationic Polymerization of Isobutyl Vinyl Ether with HCl/SnCl 4 / nBu 4 NCl 1,”Macrmolecules, 31 (15) 4703-4709.
Publisher – Google Scholar
13. El Glaoui, M., Jeanneau, E., Rzaigui, M. and Ben Nasr, C. (2009 ) “4-(3-Ammoniopropyl)morpholin-4-ium tetrachloridozincate(II),” Acta Crystallographica, E65 m282.
Publisher – Google Scholar
14. El Glaoui, M., Jeanneau, E., Lefebvre, F. and Ben Nasr, C. (2009 ) “Structural, spectroscopic and thermal investigations of a new bis(5-chloro-2,4-dimethoxyanilinium) tetrachloridocadmate trihydrate ,” Candian Journal of Analytical Sciences and Spectroscopy,54 70-81.
15. Ratajczak, H.J. (1969) “Structural studies of some hydrogen-bonded ferroelectrics using polarized ir radiation,“Journal of Molecular Structure, 3 (1-2) 27-41.
Publisher – Google Scholar
16. Navak, A. (1990 ) “Vibrational studies of structural phase transitions in partially ordered solids” Journal of Molecular Structure, 217 (1-2) 35-49.
Publisher – Google Scholar
17. Smirani, W., Ben Nasr, C. and Rzaigui, M. (2004) “Synthesis and crystal structure of a new o-ethylphenylammonium triphosphate [2-C2H5C6H4NH3]3H2P3O10,” Materials Research Bulletin, 39 (7-8) 1103-1111.
Publisher – Google Scholar
18. Kaabi, K., Rayes, A., Ben Nasr, C., Rzaigui, M. and Lefebvre, F. (2003)” Synthesis and crystal structure of new dihydrogenomonophosphate (4-C2H5C6H4NH3)H2PO4,” Materials Research Bulletin, 38 (5) 741-747.
Google Scholar
19. Oueslati, A., Ben Nasr, C., Durif, A. and Lefebvre, F. (2005) “Synthesis and characterization of a new organic dihydrogen phosphate-arsenate: [H2(C4H10N2)][H2(As, P)O4]2,” Materials Research Bulletin, 40 (6) 970-980.
Publisher – Google Scholar
20. Oueslati, A., Rayes, A., Ben Nasr, C. and Lefebvre, F. (2005) “Synthesis and characterization of 2-amino-3-methylpyridinium dihydrogenomonoarsenate,” Materials Research Bulletin, 40 (10) 1680-1689.