Introduction
Glucocorticoids are commonly used in combination with other immunosuppressive agents, including calcineurin inhibitors and mycophenolate, for the prevention and treatment of transplant rejection. Their use can be accompanied by hyperglycaemia which may worsen pre-existing diabetes or precipitate new onset diabetes after transplantation (NODAT). Mechanisms of glucocorticoid induced hyperglycaemia are multifactorial and the resultant hyperglycaemia is likely to vary depending on the type and frequency of its administration 1-6.
Studies in renal transplant recipients have shown that between 2 and 50% develop NODAT, increasing morbidity and mortality and reducing graft survival 7-13. This variability in incidence is thought to be due to lack of standardised definitions or diagnostic criteria used in early studies, short observation periods, varying therapeutic regimens and differing prevalence of diabetes in different study populations. The risk also appears to increase with higher daily doses of glucocorticoids. Recent data suggest that NODAT incidence increases over time: 10%-20% at 1 year, 20%-25% at 3 years, and 25%-30% at 10 years post-transplant 14-17. A recent study has also shown that hyperglycaemia occurs commonly immediately post-transplant in non-diabetic kidney recipients and that higher blood glucose values in the early post-transplant period may identify patients at risk of developing NODAT 18.
Few studies of optimal strategies to diagnose and manage hyperglycaemia, during the use of glucocorticoids and other diabetogenic immunosuppressive agents, are found in the literature and little evidence exists to quantify the time of day and length of the resultant hyperglycaemic excursions. Continuous glucose monitoring (CGM) provides a new method of obtaining comprehensive glucose profiles and providing information regarding the direction, magnitude, duration and frequency of fluctuations in blood glucose levels 19-21. It allows the identification of trends, often unnoticed with intermittent blood glucose and HbA1c measurements and provides information regarding nocturnal hypoglycaemia and postprandial hyperglycaemia 22-23.
Accurate detection of blood glucose patterns could assist in determining the type and timing of treatment required and may also be used to identify the best time for finger-prick blood glucose monitoring. This study aimed to explore the occurrence, timing and distribution of glucose excursions by CGM in kidney transplant recipients receiving glucocorticoids, and confirm whether they exhibit consistent diurnal variation by analysing their 24 hour glucose profiles.
Research Design and Methods
Participants receiving oral glucocorticoid therapy post-transplantation were recruited from the Royal Prince Alfred Hospital Renal Transplant Unit. Inclusion criteria for this study included individuals who have undergone recent organ transplantation (new transplant recipients) and were about to commence or already receiving steroid therapy. Exclusion criteria included patients aged less than 18 years, individuals with known diabetes prior to transplant and participants who were expected to be on glucocorticoid treatment for less than 3 months. The study protocol was approved by the Institutional Ethics Review Board at Royal Prince Alfred Hospital (Protocol no.: X09-0011 and HREC/09/RPAH/15). Informed consent was obtained according to our institutional ethics review committee policy.
Study Design
All subjects had a fasting venous blood glucose measurement prior to commencing the study. They performed self-monitoring of blood glucose (SMBG) by intermittent capillary blood sampling, using a glucometer and underwent continuous glucose monitoring for 72 hours, using a portable Medtronics Guardian® REAL-Time continuous glucose monitoring system (CGMS) (Minimed, Northbridge, California). Medtronics CGM uses a glucose oxidase-based sensor to measure interstitial glucose in subcutaneous tissue.
A wireless sensor was inserted subcutaneously into the participant’s abdomen, measuring interstitial glucose levels every 5 minutes for 72 hours and sending it to a monitor for storage. Patients were given written instructions and trained in its use. All patients also received home blood glucose meter training to perform capillary blood glucose measurements four times daily to calibrate the sensor. After the removal of the sensor, the stored information was downloaded onto a computer for further analysis.
Compared with intensive conventional blood glucose measurements, continuous blood glucose monitoring provides much greater insight into glucose levels and trends, facilitating detailed research studies of 24-hour glucose profiles.
Diagnosis and classification of diabetes was based on the current WHO diagnostic criteria of fasting plasma glucose ≥7.0mmol/L or random plasma glucose >11.0mmol/L. The post-meal value was defined as the value obtained 2 hours after the pre-meal measurement.
Statistical Methods
Data were analysed using NCSS (Number Cruncher Statistical System, Kaysville, Utah) 2007. CGM recordings were grouped into 4 equal time periods (Morning: 6am-12pm, Afternoon: 12pm-6pm, Evening: 6pm-12am, Night: 12am-6am). BGLs were determined every 5 minutes by CGM and, hourly means were used to determine GlucoseAUC (GAUC) of each period and its % increase above the Night values.
Results were expressed as mean+SD or median[IQR] and compared by Kruskal-Wallis test. Multivariate analysis was used to assess which factors determined the magnitude of rise in each of the three day time quarters.
Independent variables included diabetes status, prednisone dosage, types of immunosuppressive therapy (in addition to prednisone), the different time periods, fasting BGL and Night GAUC. Only significant variables were included in the final model. Area under the curve was calculated using the trapezoidal rule. Statistical significance level was accepted at p<0.05.
Results
CGM was used successfully and appropriately by all patients 3-10 weeks post-transplantation and sensor insertion was well tolerated. One patient was excluded as she failed to measure BGLs for calibration.
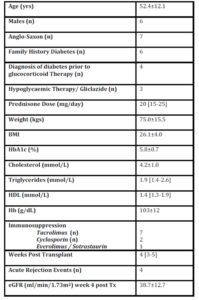
Ten patients (6 males/4 female), aged 52.4 12.1years and with a body mass index(BMI) of 26.1 4.0kg/m2 were treated with daily morning doses of prednisone ranging from 15mg to 25mg (mean:19.75 4.15mg/day). One had longstanding diabetes and a further three had new onset diabetes treated with a sulphonylurea prior to undergoing CGM. Six participants had a family history of diabetes.
All patients were also receiving other forms of immunosuppression, following recent renal transplantation (Table 1).
Table 1: Patient Demographic and Clinical Profile
Average glucose recording time was 65 hours 32 minutes. Blood glucose was measured 280+/-20 times per 24 hour period and on average 784+/-49 total glucose measurements were recorded per patient. Analysis showed hyperglycaemia was most severe in the Afternoon period. Night GAUC was associated with the magnitude of rise in GAUC for the three other time periods (p<0.002) (Table 2). The presence of established diabetes was a significant determinant of the magnitude of rise in GAUC in both the Morning and Afternoon Periods (p<0.02).
Table 2: Magnitude of GAUC and its % increase above Night GAUC
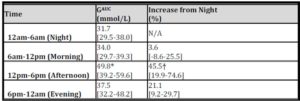
(* vs Night , † vs Morning, p<0.002)
Only one of the ten patients experienced an episode of hypoglycaemia (blood glucose level <3.3mmol/L). In this case, hypoglycaemia was observed overnight on only a single occasion with no symptoms and no causative or precipitating factors noted.
Discussion
Although research in kidney recipients is lacking, studies in the general population (including the DCCT and UKPDS) have demonstrated the beneficial effect of early intensive glycaemic control in preventing complications in type 1 and type 2 diabetes 24-28. This is of increasing importance in transplant recipients who are now living longer due to improved therapy. A need therefore exists for early diabetes detection strategies and management protocols for patients who undergo organ transplantation.
CGM offers the capability of documenting frequency, duration, time and severity of hyperglycaemic episodes more clearly than does intermittent glucose testing. Our study using CGM not only supports the limited data from earlier studies suggesting that patients receiving mane oral prednisone together with other immunosuppressive agents exhibited a maximal increase in blood glucose during the afternoon 29-32 but was also able to quantify glucose excursions more accurately, noting that the afternoon excursion is present for a longer period (Figure 1).

Figure 1: CGM profile of a typical kidney transplant recipient at day 28 post-transplant receiving tacrolimus, mycophenolate and prednisone for immunosuppression
This helps to define the degree of anti-hyperglycaemic therapy that should be directed to this period of time. Despite our modest sample size which is a limitation of this study, and CGM limited to 72 hours, this is the first study of its kind in renal transplant recipients to continuously evaluate the timing and magnitude of glucose excursions during the day and overnight. Future studies incorporating a larger sample size would allow for multivariate analysis to confirm that the negative associations were real. Further studies addressing the impact of various therapies on BGL control and the modulating effects of immunosuppressive agents are warranted.
Although CGM is well tolerated and highly informative, its use to screen for hyperglycaemia for every patient is not practical, as it is expensive and requires additional training. However, a study of this nature helps to provide quantitative data to guide evidence based management.
In conclusion, patients receiving oral prednisone in the morning exhibited a maximal increase in blood glucose during the afternoon. In patients receiving mane prednisone, BGL measurement in the afternoon would be more sensitive in detecting hyperglycaemia and guiding the need for anti-hyperglycaemic therapy, with measurement of fasting BGL alone clearly an inadequate measure.
Treatment should therefore target improving insulin availability, particularly in the afternoon. The persistence of hyperglycaemia throughout the afternoon period suggests that short acting insulin with a 4 to 6 hour action, rather than a rapid acting insulin analogue, may provide better treatment for this type of hyperglycaemia. In subjects with pre-existing diabetes and overnight hyperglycaemia, additional measures to provide more insulin in the morning and evening may also be necessary.
Further studies addressing the impact of various therapies on BGL control and the modulating effects of immunosuppressive agents over a broader range of corticosteroid type and dosage are warranted
References
1. Pagnano G, Cavallo-Perin P, Cassader M, Bruno A, Ozzello A, Masciola P. An in vivo and in vitro study of the mechanisms of prednisone-induced insulin resistance in healthy subjects. Journal of Clinical Investigation. 1983; 72(5): 1814–1820.
Publisher – Google Scholar
2. Trence DL. Management of patients on chronic glucocorticoid therapy: an endocrine perspective. Primary Care. 2003; 30(3):593-605.
Google Scholar
3. Miller MB, Neilson J. Clinical Features of the diabetic syndrome appearing after steroid therapy. Postgrad Med J. 1964; 40:660-9.
Publisher – Google Scholar
4. Hirsch IB, Paauw DS. Diabetes management in special situations. Endocrinol Metab Clin North Am. 1997; 26(3):631-45.
Publisher – Google Scholar
5. Chan JC, Cockram CS. Drug-induced disturbances of carbohydrate metabolism. Adverse Drug React Toxicol Rev. 1991; 10:1-29.
Google Scholar
6. Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J Clin Endocrinol Metab. 1982 ;54(1):131-8.
Publisher – Google Scholar
7. Bloom RD, Crutchlow MF. New-onset diabetes mellitus in the kidney recipient: diagnosis and management strategies. Clin J Am Soc Nephrol. 2008; 3 Suppl 2:S38-48
Publisher – Google Scholar
8. Davidson, J, Wilkinson, A, Dantal, J, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003; 75:SS3-SS24.
Google Scholar
9. Caillard S, Eprinchard L, Perrin P, Braun L, Heibel F, Moreau F, Kessler L, Moulin B. Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematic screening by oral glucose tolerance test. Transplantation. 2011; 15;91(7):757-64.
Google Scholar
10. Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC:
‘Posttransplantation diabetes: A systematic review of the literature. Diabetes Care. 2002; 25:583 –592.
Publisher – Google Scholar
11. Cosio, FG, Pesavento, TE, Kim, S, et al. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 2002; 62:1440-1446.
Google Scholar
12. Vesco, L, Busson, M, Bedrossian, J, et al. Diabetes mellitus after renal transplantation: characteristics, outcome, and risk factors. Transplantation 1996; 61:1475-1478.
Publisher – Google Scholar
13. Friedman, EA, Shyh, TP, Beyer, MM, et al. Posttransplant diabetes in kidney transplant recipients. Am J Nephrol 1985; 5:196-202.
Publisher – Google Scholar
14. Bloom RD, Crutchlow MF. Transplant-associated hyperglycemia. Transplantation Reviews 22 (2008) 39–51.
Publisher – Google Scholar
15. Kasiske B, Snyder JJ, Gilbertson D et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3:178
Publisher – Google Scholar
16. Webster AC, Woodroffe RC, Taylor RS et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. Br Med J 2005; 331: 810
Publisher – Google Scholar
17. Chadban S. New onset diabetes after transplantation – should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients. Nephrol Dial Transplant. 2008 ;23(6):1816-8.
Publisher – Google Scholar
18. Wojtusciszyn A, Mourad G, Bringer J, Renard E. Continuous glucose monitoring after kidney transplantation in non-diabetic patients: early hyperglycaemia is frequent and may herald post-transplantation diabetes mellitus and graft failure. Diabetes Metab. 2013; 39(5):404-1
Publisher – Google Scholar
19. Derosa G, Salvadeo SA, Mereu R, D’Angelo A, Ciccarelli L, Piccinni MN, Ferrari I, Gravina A, Maffioli P, Tinelli C. Continuous glucose monitoring system in free-living healthy subjects: results from a pilot study. Diabetes Technol Ther. 2009 ;11(3):159-69.
Publisher – Google Scholar
20. Hirsch IB. Realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. Journal of Endocrinology and Metabolism. 2009; 94(7): 2232-8.
Publisher – Google Scholar
21. Hirsch IB, Armstrong D, Bergenstal RM, Buckingham B, Childs BP, Clarke WL, Peters A, Wolpert H.
Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-44
Publisher – Google Scholar
22. Zick R, Petersen B, Richter M, Haug C; SAFIR Study Group. Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with Type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9(6):483-92.
Publisher – Google Scholar
23. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24(11):1858-62.
Google Scholar
24. The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329 :977– 986,1993
25. The UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352 :837– 853,1998
Google Scholar
26. The UK Prospective Diabetes Study (UKPDS) Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet352 :854– 865,1998
Google Scholar
27. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group: Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 342 :381– 389,2000
Publisher – Google Scholar
28. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med353 :2643– 2653,2005
29. Iwamoto T, Kagawa Y, Naito Y, et al. Steroid-induced diabetes mellitus and related risk factors in patients with neurologic diseases.Pharmacotherapy. 2004; 24:508-514.
Publisher – Google Scholar
30. Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract. 2007;105:c54-c57.
31. Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011; 96(6):1789-96. Epub 2011 Mar 16.
Publisher – Google Scholar
32. Rodríguez LM, Knight RJ, Heptulla RA. Continuous glucose monitoring in subjects after simultaneous pancreas-kidney and kidney-alone transplantation. Diabetes Technol Ther. 2010; 12(5):347-51.
Publisher – Google Scholar