Our study comprises a comprehensive comparison between ischemic stroke patients with and without diabetes mellitus. One important finding was that although diabetes mellitus was more frequent among old patients, assessing the sexes separately disclosed important differences. Among males the relative frequencies of diabetes mellitus increased with increasing age up to about 75-80 years and then declined. By contrast, among females the relative frequency of diabetes mellitus was constant irrespective of age interval. Other studies have disclosed conflicting results, finding that patients with diabetes mellitus are older (Megherbi et al., 2003) (Jorgensen et al., 1994), younger (Kissela et al., 2005) or no differences according to age (Karapanayiotides et al., 2004) (Zhang et al., 2012a).
Our study shows that patients with diabetes mellitus have more traditional risk factors than patients without diabetes mellitus. Thus, prior hypertension, myocardial infarction, angina pectoris and cerebral infarction analysed separately were significantly more frequent in the diabetes mellitus group. These results are compatible to findings in other studies (Reeves et al., 2010) (Harriott et al., 2013).
Smoking was significantly less frequent among patient with diabetes mellitus, even when adjusting for age. It is likely that patients with diabetes mellitus are less prone to smoking because they are aware of the relation between smoking and diabetic complications. One study found no differences in smoking frequencies between stroke patients with and without diabetes mellitus (Megherbi et al., 2003), but another study found frequencies in agreement with our study (Giorda et al., 2007). We have recently shown that recurrent vascular events and long term mortality are highly associated with the number of traditional risk factors in patients with cerebral infarction (Gjerde and Naess, 2013),(Putaala et al., 2010). Our study shows that the risk factor burden (other than diabetes mellitus) is especially high among patients with DM and cerebral infarction. This underlines the need for aggressive secondary preventive treatment among patients with diabetes mellitus. Physical activity, low fat, low carbohydrate diet, weight loss, moderation of alcohol, careful monitoring of diabetes, hypertension and others risk factors will reduce the severity and recurrence of stroke and improve quality of life (American Diabetes, 2012),(Hewitt et al., 2012).
There was no significant difference as to stroke severity on admission between patients with and without diabetes mellitus. However, short-term outcome as measured by the mRS was significantly worse among patients with diabetes mellitus even after adjusting for confounding factors including stroke severity on admission. A possible explanation is that ischemic brain tissue is more vulnerable in patients with diabetes mellitus. Including glucose on admission in the multivariate analysis showed that short term-outcome was associated with hyperglycemia whereas now diabetes mellitus was no longer associated with outcome. This is in line with other studies that found hyperglycemia is associated with poor outcome (Bruno et al., 1999). Hyperglycemia on admission is an important determinant of infarct volume expansion, even in patients with good collateral circulation (Kimura et al., 2011),(Shimoyama et al., 2013). Hyperglycemia induces progressive cerebrovascular changes during ischemia and affects hemodynamic recovery after reperfusion (Kawai et al., 1997). Others have reported that females with diabetes mellitus have poor prognosis (Arboix et al., 2006)
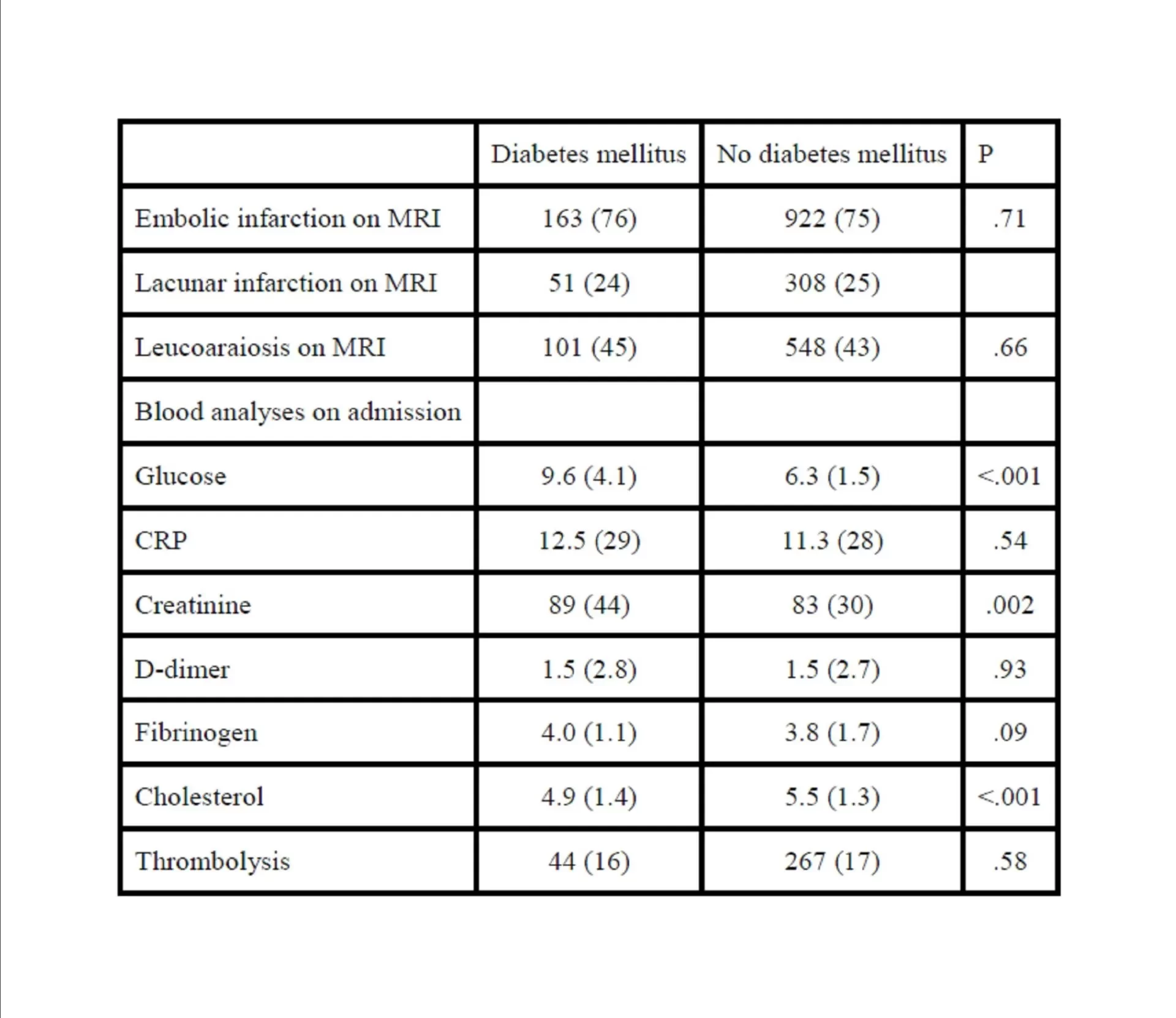
We found no differences in the distributions of ischemic stroke subtype based on the OCSP classification. This is also consistent with another study (Kiers et al., 1992). However, others have reported an association between diabetes and posterior circulation infarctions (Karapanayiotides et al., 2004) and/or lacunar infarctions (Megherbi et al., 2003),(Lees and Walters, 2005). Others have found that lacunar infarctions have better functional prognosis than embolic infarction among patients with diabetes mellitus (Arboix et al., 2001). It remains an open question whether diabetes mellitus causes small vessel disease or not.
We found that patients with diabetes mellitus had more complications than patients without diabetes mellitus during the hospital stay. Urinary retention, pneumonia and epileptic seizures were significantly more frequent among our patients with diabetes mellitus. Urinary retention was found as high as 44% in another study (Kong and Young, 2000). The authors suggested diabetic cystopathy as cause, and they believed retention to be a transient phenomenon that should be followed by postvoid residual screening in all patients immediately after stroke (Kong and Young, 2000). Another study reported high frequency of pneumonia in older patients with stroke and diabetes (30%). Older age, higher NIHSS on admission and dysphagia may predict the occurrence of pneumonia on stroke onset (Zhang et al., 2012b). Hyperglycemia may be a significant factor that induces epileptic seizures in some patients (Arboix A, 1996), (Azra Alajbegovic, 2002). We found that patients with ischemic stroke and diabetes mellitus had higher frequencies of first emergency readmission, which is supported by another study (Sun and Toh, 2009). Main diagnoses for patients readmitted within 3 months were cardio-vascular disorders, stroke related causes and infections.
Diabetes mellitus was not associated with mortality within the first 30 days after stroke onset among our patients. By contrast, another study found increased early mortality in patients with stroke and diabetes mellitus (Kiers et al., 1992). We found that among 30 days survivors long term mortality was associated with diabetes mellitus after adjusting for confounders. Another large study found that diabetic patients did not have significantly higher mortality rate at 60 days and 1 year after stroke onset, but long term mortality was higher among stroke patients with diabetes mellitus (Kamalesh et al., 2008).
One of the strengths of this study is the inclusion of patients living in a well-defined geographical region. Admission threshold is low for patients with possible acute stroke. This indicates that few cases escaped our attention. Another of the strengths of this study is access to the mortality data from the official population registry. All deaths in Norway are reported. A limitation of the study is that the cause of death was unknown. Another limitation is that we do not know the functional outcome 3 months after stroke onset.
Conclusion
Patients with diabetes mellitus and cerebral infarction have poorer short-term functional outcome, more complications, more early readmissions and higher long-term mortality than patients with cerebral infarction and without diabetes mellitus. Relative frequencies of diabetes mellitus and age differed between males and females.
Acknowledgments
The authors thank research nurse Maren Inselseth for her excellent work and assistance with data registration.
References
1. Adams, HP Jr, Bendixen, BH., Kappelle, LJ., Biller, J., Love, BB., Gordon, DL. and Marsh EE 3rd. (1993), ‘Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment,’ Stroke, 24, 35-41.
2. American Diabetes Association. (2012), ‘Standards of medical care in diabetes–2012,’ Diabetes Care, 35 (1), S11-63.
3. Arboix, A., Comes, E., Massons. J., García, L. and Oliveres M. (1996), ‘Relevance of early seizures for inhospital mortality in acute cerebrovascular disease,’ Neurology, 47, 1429-1435.
4. Arboix, A., Milian, M., Oliveres, M., García-Eroles, L. and Massons, J. (2006), ‘Impact of Female Gender on Prognosis in Type 2 Diabetic Patients with Ischemic Stroke,’ European Neurology, 56, 6—12.
5. Arboix, A., Paladilla, I., Massons J., García-Eroles, L., Comes, E., and Targa, C. (2001), ‘ Clinical study of 222 patients with pure motor stroke,’ Journal of Neurology, Neurosurgery, Psychiatry, 71 (2), 239-242
6. Aljbegovic, A., Metelko, Z., Aljbegovic, S., Kantardzic, D., Bratic, M., Suljic, E., Hrnjica, M. and
7. Resić H. (2002), ‘Correlation between early and late epileptic seizures and diabetes mellitus during and after stroke,’ Diabetologia Croatica, 31-3, 173-177.
8. Bamford, J., Sandercock, P., Dennis, M., Burn, J. and Warlow, C. (1991), ‘Classification and natural history of clinically identifiable subtypes of cerebral infarction,’ Lancet, 337 (8756), 1521-6.
9. Bruno, A., Biller, J., Adams, HP Jr., Clarke, WR., Woolson, RF., Williams, LS. and Hansen, MD. (1999), ‘Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators,’ Neurology, 52 (2), 280-4.
10. Centers for Disease Control and Prevention. (2011), ‘National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States,’ Department of Health and Human Services CfDCaP, editor. Atlanta: 2011. p. GA2011.
11. Giorda, CB., Avogaro, A., Maggini, M., Lombardo, F., Mannucci, E., Turco, S., Alegiani, SS., Raschetti, R., Velussi, M., Ferrannini, E. and DAI Study Group. (2007), ‘Incidence and risk factors for stroke in type 2 diabetic patients: the DAI study,’ Stroke, 38 (4), 1154-60.
12. Gjerde, G. and Naess, H. (2013), ‘Risk factor burden predicts long-term mortality after cerebral infarction,’ Acta Neurologica Scandinavica, 129 (3), 173-7.
13. Harriott, AM., Dueker, N., Cheng, YC., Ryan, KA., O’Connell, JR., Stine, OC., McArdle, PF., Wozniak, MA., Stern, BJ., Mitchell, BD., Kittner, SJ. and Cole, JW. (2013), ‘Polymorphisms in migraine-associated gene, and ischemic stroke risk in a biracial population: the genetics of early onset stroke study,’ Springerplus, 2 (1), 46.
14. Hewitt, J., Castilla, Guerra L., Fernández-Moreno, Mdel C. and Sierra, C. (2012),’ Diabetes and stroke prevention: a review,’ Stroke Res Treat, 2012, 673187.
15. Jia, Q., Zhao, X., Wang, C., Wang, Y., Yan, Y., Li, H., Zhong, L., Liu, L., Zheng, H., Zhou, Y. and Wang, Y. (2011), ‘Diabetes and poor outcomes within 6 months after acute ischemic stroke: the China National Stroke Registry,’ Stroke, 42 (10), 2758-62.
16. Jørgensen, HS., Nakayama, H., Raaschou, HO. and Olsen, TS. (1994), ‘Effect of blood pressure and diabetes on stroke in progression,’ Lancet, 344 (8916), 156-9.
17. Kamalesh, M., Shen, J. and Eckert, GJ. (2008), ‘Long term postischemic stroke mortality in diabetes: a veteran cohort analysis,’ Stroke, 39 (10), 2727-31.
18. Karapanayiotides, T., Piechowski-Jozwiak, B., van Melle, G., Bogousslavsky, J. and Devuyst, G. (2004), ‘Stroke patterns, etiology, and prognosis in patients with diabetes mellitus,’ Neurology, 62 (9), 1558-62.
19. Kawai, N., Keep, RF. and Betz, AL. (1997), ‘Hyperglycemia and the vascular effects of cerebral ischemia,’ Stroke, 28 (1), 149-54.
20. Kiers, L., Davis, SM., Larkins, R., Hopper, J., Tress, B., Rossiter, SC., Carlin, J. and Ratnaike. S. (1992), ‘Stroke topography and outcome in relation to hyperglycaemia and diabetes,’ Journal of Neurology, Neurosurgery & Psychiatry, 55 (4), 263-70.
21. Kimura, K., Sakamoto, Y., Iguchi, Y., Shibazaki, K., Aoki, J., Sakai, K. and Uemura, J. (2011), ‘Admission hyperglycemia and serial infarct volume after t-PA therapy in patients with and without early recanalization,’ Journal of Neurological Sciences, 307 (1-2), 55-9.
22. Kissela, BM., Khoury, J., Kleindorfer, D., Woo, D., Schneider, A., Alwell, K., Miller, R., Ewing, I., Moomaw, CJ., Szaflarski, JP., Gebel, J., Shukla, R. and Broderick, JP. (2005), ‘Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study,’ Diabetes Care, 28 (2), 355-9.
23. Kong, KH. and Young, S. (2000). Incidence and outcome of poststroke urinary retention: a prospective study,’ Archives of Physical Medicine and Rehabilitation, 81 (11), 1464-7.
24. Lees, KR. and Walters, MR. (2005), ‘Acute stroke and diabetes,’ Cerebrovascular Diseases, 20 Suppl 1, 9-14.
25. Lindley, RI., Warlow, CP., Wardlaw, JM., Dennis, MS., Slattery, J. and Sandercock, PA.
26. (1993), ‘Interobserver reliability of a clinical classification of acute cerebral infarction,’ Stroke, 24 (12), 1801-4.
27. Megherbi, SE., Milan, C., Minier, D., Couvreur, G., Osseby, GV., Tilling, K, Di Carlo, A., Inzitari, D., Wolfe, CD., Moreau, T., Giroud, M. and European BIOMED Study of Stroke Care Group. (2003), ‘Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project,’ Stroke, 34 (3), 688-94.
28. Phipps, MS., Jastreboff, AM., Furie, K. and Kernan, WN. (2012), ‘The diagnosis and management of cerebrovascular disease in diabetes,’ Current Diabetes Reports, 12 (3), 314-23.
29. Putaala, J., Haapaniemi, E., Metso, AJ., Metso, TM., Artto, V., Kaste, M. and Tatlisumak, T. (2010), ‘Recurrent ischemic events in young adults after first-ever ischemic stroke,’ Annals of Neurology, 68 (5), 661-71.
30. Reeves, MJ., Vaidya, RS., Fonarow, GC., Liang, L., Smith, EE., Matulonis, R., Olson, DM., Schwamm LH., Get With The Guidelines Steering Committee and Hospitals. (2010), ‘Quality of care and outcomes in patients with diabetes hospitalized with ischemic stroke: findings from Get With the Guidelines-Stroke,’ Stroke, 41 (5), e409-17.
31. Rohr, J., Kittner, S., Feeser, B., Hebel, JR., Whyte, MG., Weinstein, A., Kanarak, N., Buchholz, D., Earley, C., Johnson, C., Macko, R., Price, T., Sloan, M., Stern, B., Wityk, R., Wozniak, M. and Sherwin, R. (1996), ‘Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington Cooperative Young Stroke Study,’ Archives of Neurology, 53 (7), 603-7.
32. Shimoyama, T., Shibazaki, K., Kimura, K., Uemura, J., Shiromoto, T., Watanabe, M., Inoue, T., Iguchi, Y. and Mochio, S. (2013), ‘Admission hyperglycemia causes infarct volume expansion in patients with ICA or MCA occlusion: association of collateral grade on conventional angiography,’ European Journal of Neurology, 20 (1), 109-16.
33. Sun, Y. and Toh, MP. (2009), ‘Impact of diabetes mellitus (DM) on the health-care utilization and clinical outcomes of patients with stroke in Singapore,’ Value Health, 12 Suppl 3, S101-5.
34. Wahlgren, N., Ahmed, N., Dávalos, A., Ford, GA., Grond, M., Hacke, W., Hennerici, MG., Kaste, M., Kuelkens, S., Larrue, V., Lees, KR., Roine, RO., Soinne, L., Toni, D., Vanhooren, G. and SITS-MOST investigators. (2007), ‘Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study,’ Lancet, 369 (9558), 275-82.
35. Wessels, T., Wessels, C., Ellsiepen, A., Reuter, I., Trittmacher, S., Stolz, E. and Jauss, M. (2006), ‘Contribution of diffusion-weighted imaging in determination of stroke etiology,’ AJNR American Journal of Neuroradiology, 27 (1), 35-9.
36. Zhang, B., Gao, C., Hou, Q., Yin, J., Xie, L., Pu, S., Yi, Y. and Gao, Q. (2012), ‘The potent different risk factors for cerebral infarction in young patients with and without type 2 diabetes: subanalysis of the Young Cerebral Infarction Study (YCIS),’ Atherosclerosis, 221 (1), 215-20.
37. Zhang, X., Wang, F., Zhang, Y. and Ge, Z. (2012), ‘Risk factors for developing pneumonia in patients with diabetes mellitus following acute ischaemic stroke,’ Journal of International Medical Research, 40 (5), 1860-5.