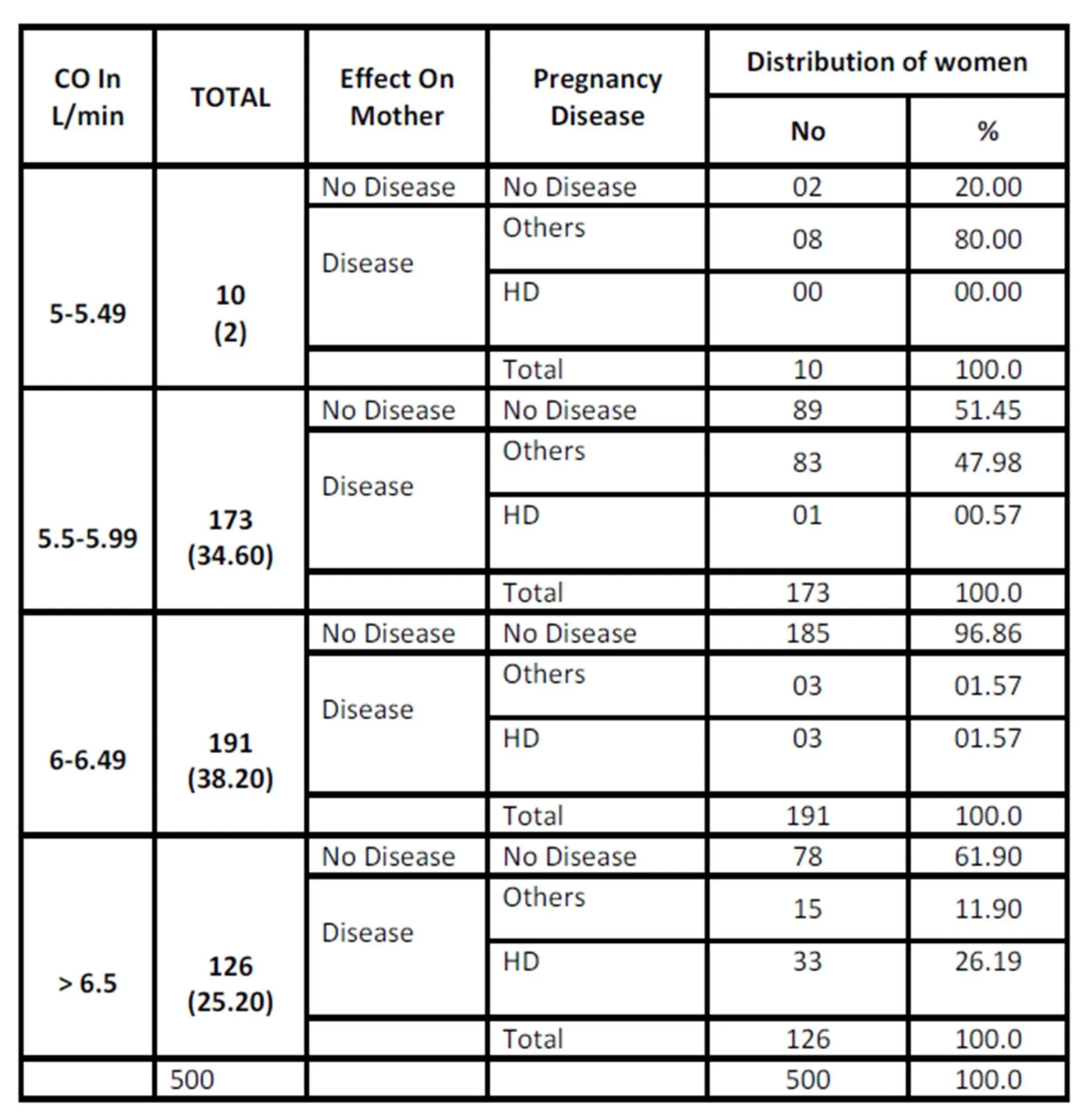
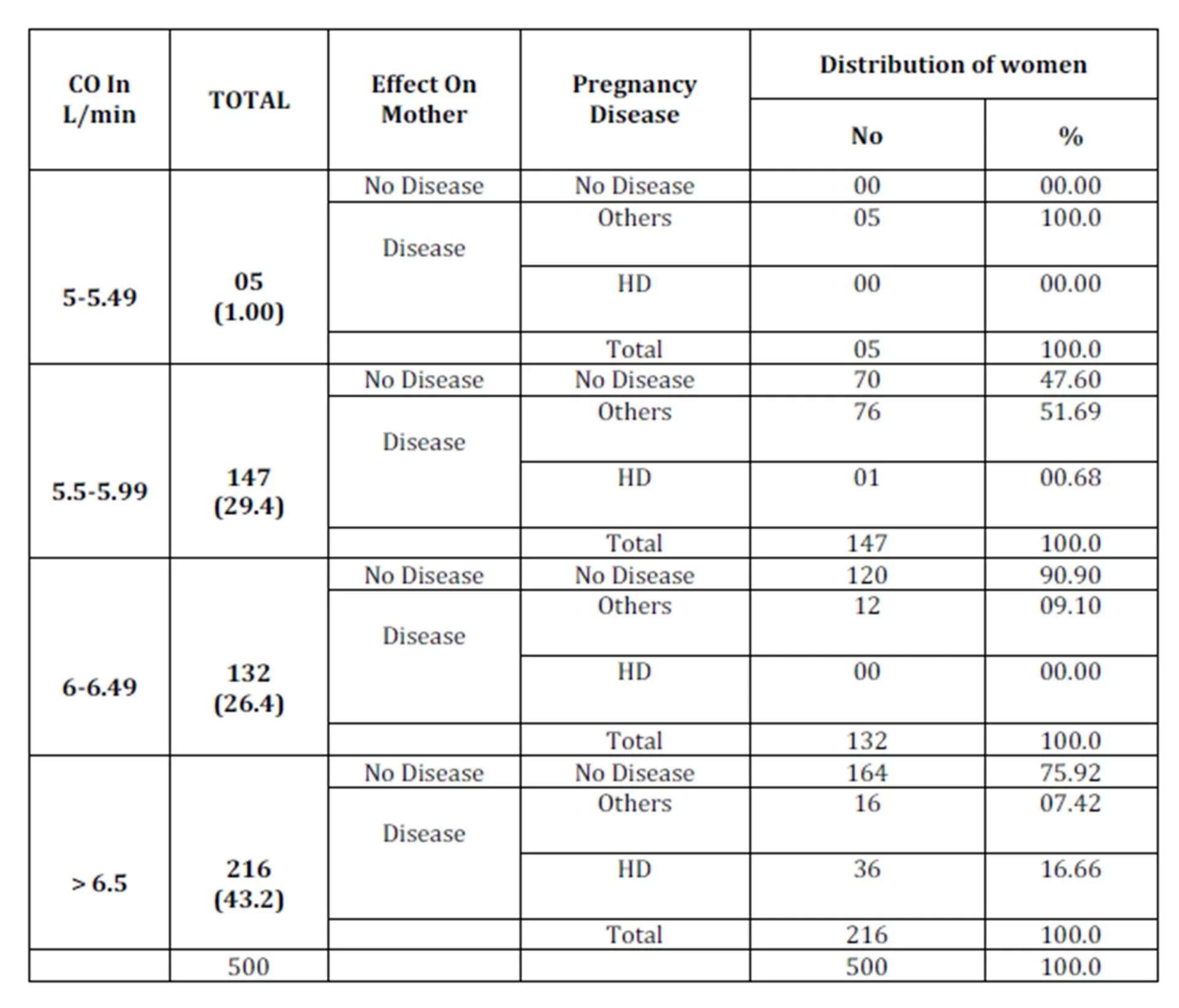
HD=Hypertensive Disorder, CO = Cardiac Output
Cardiac output was not affected by residence and age at any gestation ( P value at all gestations was >0.05.), no significant difference (P value >0.05 )in mean CO at different gestations in relation to parity and also no significant difference was seen with age ,parity and mean CO at different gestations as P value at different gestations was >0.05.
There was no significant difference in mean CO at different gestations in relation to BMI (P value >0.05). Also, there was no significant difference (P value >0.05) in mean CO at different gestations in relation to age, BMI. No significant difference (P value >0.05) was seen in mean CO at different gestations in relation to age, weight.
Overall, the CO at 10 ± 2 weeks had a high degree of correlation with cardiac output at increasing gestation (P= < 0.001), and the predicted cardiac output with increasing gestation could be estimated using analysis of variance of cardiac output at 10 ± 2 weeks. Overall, women who had high cardiac output in early pregnancy were more prone to develop hypertensive disorders.
Discussion
As of present times, the ultimate primary prevention of hypertensive disorders of pregnancy is avoidance of pregnancy. Secondary prevention of the disease requires knowledge of the patho-physiological mechanism and modality of prevention. If the disease cannot be prevented, there should be available, usable techniques for the detection of disorder at early stage and after the detection, intervention of the disease process is essential [9-10]. But for the prevention of the disease, its severity and death due to the disease, prediction is necessary, which has limitations as of now, so the research continues. Cunningham reports that the cardio- vascular hemodynamics of the women with severe preeclampsia are characterized by normal or reduced CO and elevated vascular resistance [6]. However, Easterling had earlier conducted a study designed to test the hypothesis that preeclampsia is associated with high cardiac output, where in researchers measured the CO using Doppler during pregnancy and postpartum in women with preeclampsia and concluded that CO was elevated throughout pregnancy in women who later developed preeclampsia [11]. At 6 weeks postpartum, the cardiac output was elevated though the hypertension was resolved (p = 001) and peripheral resistance remained lower than in the normotensive subjects (p = 001).
In a longitudinal study of 400 primigravidae studied with doppler echocardiography by Bosio et al, the researchers reported that the gestational hypertension developed in 24 (6.34%) women and preeclampsia in 20 (5.29%) women out of the 378 women who completed the pregnancy [7]. Women with preeclampsia had significantly elevated CO before clinical diagnosis of hypertensive compared to normotensive controls, but total peripheral resistance was not significantly different during this latent phase. Study findings supported the hyperdynamic disease model with a subsequent crossover to low cardiac output state.
In the study by De Paco et al [12,] CO was significantly higher in the preeclampsia and PIH cases, and in these cases alterations in maternal CO predated the clinical onset of the disorders by several months. Maternal CO in the first trimester was found to be increased in women who developed preeclampsia later. Most of the research and studies about the relationship with cardiac output have been in patients with hypertensive disorders, and the studies on normal pregnancies for prediction are scarce. When prediction is possible, then only an intervention can be thought of. Easterling conducted an interventional study to determine if the assessment of maternal hemodynamics could predict women at risk for the development of preeclampsia, whether treatment directed at hemodynamic abnormalities before the onset of hypertension could prevent hypertensive disorders of pregnancy [13]. Nulliparous and diabetic subjects at risk were treated with 100 mg of atenolol or placebo. Researchers concluded that women at risk for hypertensive disorders of pregnancy can be identified by the measurement of CO in the second trimester, and treatment with atenolol decreased the occurrence of hypertensive disorders. Carr did a study to evaluate whether women with risk for disorders had a hyperdynamic circulation, and concluded that women with clinical risk factors had hyperdynamic circulation in the early second trimester compared with women at low risk [8]. These women also had evidence of increased endothelial activation as assessed by measuring plasma vascular cell adhesion molecule.
Tihtonen conducted a study assessing haemodynamic parameters by whole-body impedance cardiography noninvasively with the purpose of determining how hypertensive disorders of pregnancy modify maternal haemodynamics [14]. In women with hypertensive disorders of pregnancy, there was higher systemic vascular resistance index (SVRI), mean arterial pressure (MAP) and lower stroke index (SI) and cardiac index (CI), thus the baseline haemodynamics was significantly different in women with hypertensive disorders from healthy women.
In the present study, out of total 500 women studied, 37 (7.4%) developed hypertensive disorders. Their mean CO at 10 ± 2 weeks gestation was 5.77 L/min which increased to 6.94 L/min at 30 ± 2 weeks. Women who had low cardiac output in early pregnancy had less risk of developing hypertensive disorders, while women who had high CO in the early pregnancy were at increased risk of developing hypertensive disorders later; significant relation was seen between high maternal CO and hypertensive disorders.
The present study of prediction of hypertensive disorders by estimating CO from early pregnancy reveals that, no significant difference was seen with age, parity, height, weight and BMI and mean CO at different gestations.
In the present study of prediction of hypertension disorders by estimating the CO from early pregnancy reveals that, the CO at 10 ± 2 weeks has positive and high degree of correlation with hypertensive disorders of pregnancy (P=<0.001), and CO was maximum at 22± 2 weeks and CO at 10± 2 weeks was predictor of CO at 30± 2 weeks and a significant relation was found between high maternal CO and hypertensive disorders.
An attempt has been made in the present study for the prediction of hypertensive disorders by estimating CO from early pregnancy. Conclusions drawn from the study also include no significant relation between CO at different gestation with age, parity, height, weight and BMI, and women who had high cardiac output in early pregnancy were more prone to develop hypertensive disorders.
Acknowledgement
Authors are grateful to the women who agreed to participate and to everyone in the Department of physiology, especially the head of the department for the help.
Disclosure Policy
Competing interest none.
References
1. Pipkin FB, Chamberlain G, Steer PJ.Maternal physiology in pregnancy, Turnbulls Obstetrics, third edition Churchil Livingstone, Sydney , Toronto 2001; 71-91.
2. O’Toole, M.L. Physiologic aspects of exercise in pregnancy. Clin Obstet Gynecol. 2003., 46(2):379-389.
Publisher – Google Scholar
3. Foley MR, Maternal cardiovascular and hemodynamic adaptation to pregnancy, Uptodate patient information. 2007; 15,3.
4. Chapman AB, Abraham WT, Zamudio S Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998; 54; 2056-2063.
Publisher – Google Scholar
5. David P, Maternal cardiac disease, Developing Anaesthesia.org 2007.
6. Cunningham F, Gant N, Gilstrap L, Hauth J., Wenstrom K. Hypertensive disorder in pregnancy. In Williams Obstetrics 21th ed. The McGraw-Hill Companies, Singapore, 2003. 567-617.
7. Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999; 94:978—984.
Publisher – Google Scholar
8. Carr DB, McDonald GB, Brateng D, Desai M, Thach T, Easterling TR. The relationship between hemodynamics and inflammatory activation in women at risk for preeclampsia. Obstetrics & Gynecology 2001; 98:1109-1116.
Publisher – Google Scholar
9. Barker, D. J. “The developmental origins of well-being.” Philos Trans R Soc Lond B Biol Sci 2004: 359(1449): 1359-1366.
Publisher – Google Scholar
10. Dekker, G. and Sibai B. “Primary, secondary, and tertiary prevention of pre-eclampsia.” Lancet . 2001: 357(9251): 209-215.
Publisher – Google Scholar
11. Easterling T Carr DB, McDonald GB, Brateng D, Desai M, Thach T, Easterling TR.The relationship, Benedetti T, Schmucker B, Millard S. Maternal hemodynamics in normal and preeclamptic pregnancies. A longitudinal study.Obstet Gynecol. 1990;76: 1061—9.
Google Scholar
12. De Paco C, Kametas N, Rencoret G, Strobl I, Nicolaides KH. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Doi: 10.1097/01.AOG.0000298622.22494.0c. 2008;111 (2 Pt 1): 292-300
Publisher – Google Scholar
13. Easterling T, Brateng D, Schmucker B, Brown Z, Millard S. Prevention of preeclampsia: A randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999; 93:725—33.
Publisher – Google Scholar
14. TihtonenKati ,Maternalhaemodynamics in hypertensive and normotensive pregnancy, ActaUniversitatis Tamperensis; 2006; 1189.
Google Scholar