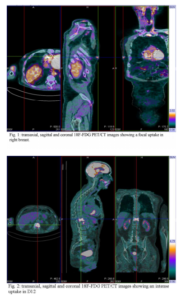
The patient underwent further examinations: high Ca15.3 levels were found (275 UI/ml) and lesions in breast and spine were pathologically confirmed as ductal breast carcinoma. In particular, there was an evidence of ductal structures for both mammalian and metastatic lesions, which also had prominent nuclei, high mitotic rate (Ki67>70%) and were positive to HER2.
The patient was also investigated about genetic mutation and was found to be positive for mutation in BRCA2 gene.
Discussion
Patients affected by parathyroid tumors are at increased risk of developing second malignancies and breast as been demonstrated as having a 1.2 standardized incidence ratios in women affected by parathyroid adenoma in a study by Fallah et al.; anyway, risk of other cancers after parathyroid adenocarcinoma has been observed not to increase in the same study.
How some proteins which are overexpressed in hyperparathyroidism can increase risk of developing breast cancer has been recently investigated in many papers.
In particular, a paper by Liang et al showed that upregulation of type 1 receptor parathyroid hormone (PTH1R), associated with high levels of parathyroid hormone (PTH) are responsible of an increased proliferation and apoptosis of breast cancer cells .
Another paper by Ibaragi demonstrated that parathyroid hormone-related protein (PTHrP) might have roles in breast cancer bone metastasis independently of its roles in osteoclastic function.
Finally, also alterations of calcium serum levels have been described by Almquist as correlated with a higher risk of developing breast cancer in premenopausal and overweight women.
Taken together, all these data suggest that a pathophysiological role of endocrinological alterations observed in hyperparathyroidism, whose nature is still under active investigation, can be hypothesized in order to explain the increased risk of developing breast cancer.
The widespread bone metastates in our Patient could also be a consequence of the unbalance of many factors, caused by hyperparathyroidism.
In our case, also the fact that the patient was positive for BRCA2 gene mutation, as described for example in a review by Ghataorhe et al. and the therapy with bicalutamide could have also had a role in developing the second primary malignancy.
As described in many papers, i.e by Sieber and Chianakwalam, bicalutamide has been reported as causing an increased risk of breast events, even if a direct relationship between this therapy and breast cancer has not been demonstrated.
Anyway, there is an increasing interest in the use of prophylactic Tamoxifen therapy: as an example, a paper by Tunio suggests that Tamoxifen could be adopted in Patients treated with Bicalutamide with drug-induced gynecomastia.
To our knowledge, no cases of breast cancer in men treated with bicalutamide for a parathyroid carcinoma with high levels of androgen receptors exist in literature.
This could both support other works which highlighted the increased risk of second malignancies in patients with parathyroid carcinoma, and suggest the opportunity of starting a tamoxifen therapy in order to decrease risk of breast events.
References
Adam, M. A., Untch, B. R. & Olson, J. A. (2010). “Parathyroid Carcinoma: Current Understanding and New Insights into Gene Expression and Intraoperative Parathyroid Hormone Kinetics,” Oncologist;15: 61—72.
Publisher – Google Scholar
Almquist, M., Bondeson, A. G., Bondeson, L., Malm, J. & Manjer, J. (2010). “Serum Levels of Vitamin D, PTH and Calcium and Breast Cancer Risk–A Prospective Nested Case—Control Study,” International Journal of Cancer; 127: 2159—2168.
Publisher – Google Scholar
Chianakwalam, C. I., McCahy, P. & Griffiths, N. J. (2005). “A Case of Male Breast Cancer in Association with Bicalutamide-Induced Gynaecomastia,” Breast;14:163-4.
Publisher – Google Scholar
Fallah, M., Kharazmi, E., Sundquist, J. & Hemminki, K. (2011). “Nonendocrine Cancers Associated with Benign and Malignant Parathyroid Tumors,” Journal of Clinical Endocrinology and Metabolism; 96:E1108—E1114.
Publisher – Google Scholar
Ghataorhe, P., Kurian, A. W., Pickart, A., Trapane, P., Norton, J. A., Kingham, K. & Ford, J. M. (2007). “A Carrier of both MEN1 and BRCA2 Mutations: Case Report and Review of the Literature,” Cancer Genetics and Cytogenetics;179(2):89-92.
Publisher – Google Scholar
Ibaragi, S., Shimo, T., Iwamoto, M. et al. (2010). “Parathyroid Hormone-Related Peptide Regulates Matrix Metalloproteinase-13 Gene Expression in Bone Metastatic Breast Cancer Cells,” Anticancer Research;30:5029-36.
Publisher – Google Scholar
Liang, H., Zhong, Y., Huang, Y. & Chen, G. (2012). “Type 1 Receptor Parathyroid Hormone (PTH1R) Influences Breast Cancer Cell Proliferation and Apoptosis Induced by High Levels of Glucose,” Medical Oncology; 29:439—445.
Publisher – Google Scholar
Nilsson, I. L., Wadsten, C., Brandt, L., Rastad, J. & Ekbom, A. (2004). “Mortality in Sporadic Primary Hyperparathyroidism: Nationwide Cohort Study of Multiple Parathyroid Gland Disease,” Surgery;136:981—987.
Publisher – Google Scholar
Norenstedt, S., Granath, F., Ekbom, A. et al. (2011). “Breast Cancer Associated with Primary Hyperparathyroidism: A Nested Case Control Study,” Clinical Epidemiology; 3:103-6.
Publisher – Google Scholar
Sieber, P. R. (2007). “Treatment of Bicalutamide-Induced Breast Events,” Expert Review of Anticancer Therapy;7:1773-9.
Publisher – Google Scholar – British Library Direct
Tanaka, Y. (2010). “Primary Hyperparathyroidism with Breast Carcinoma,” Breast cancer; 17:265-8.
Publisher – Google Scholar
Tunio, M. A., Al-Asiri, M., Al-Amro, A., Bayoumi, Y. & Fareed, M. (2012). “Optimal prophylactic and Definitive Therapy for Bicalutamide-Induced Gynecomastia: Results of a Meta-Analysis,” Current Oncology;19: e280-8.
Publisher – Google Scholar