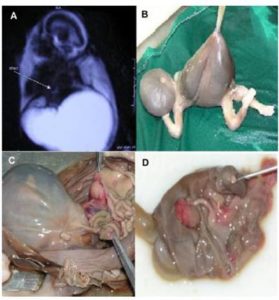
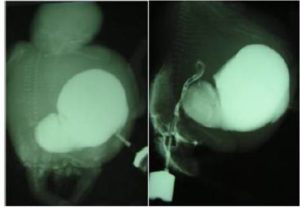
Discussion
The etiology of CDS is not exactly clear. Both genetic and environmental factors can induce this complex malformation, but there is no definitive gene identified in producing this combination of multiple developmental abnormalities (Liang, Ioffe & Sun 1998). The consumption of doxylamine succinate and etretinate (a long-acting retinoid in mice); as environmental agents, have been suggested for its occurrence (Liang, Ioffe & Sun 1998). In the past, it was considered that CDS occurred only in females; but it has been recognized that male fetuses can also be affected by this abnormality (Pauli 1994). A major characteristic of this malformation concludes absence of a median perineal raphe (Liang, Ioffe & Sun 1998). Proximal urethral dilation, oligohydramnios, enormously enlarged bladder, bilateral hydronephrosis, megacystitis, bilateral MCDK, hydroureter, various degrees of cystic dysplasia of kidneys and reduction of amniotic fluid index are the consequences of the urinary tract obstruction (Sahinoglu et al. 2004). As the time of urinary obstruction prolongs, the severity of kidney disease and pulmonary hypoplasia increases and the volume of amniotic fluid reduces, that can notably influence the neonatal survival (Sahinoglu et al. 2004). Pulmonary hypoplasia is the most frequent complication of CDS which may occur if oligohydramnios develops before 24 weeks of gestation as the result of urinary tract obstruction. In order to facilitate the prenatal counseling, the affected organs should be examined preciously by the application of more sensitive diagnostic tools. In some situations, termination of gestation is recommended; but the survival of fetuses in neonatal period and the management of these cases should also be considered. Delivery of such cases may be complicated and should be performed at a center with available perinatal, neonatal and surgical services.
One of the elements determining survival of a fetus with cloacal anomalies is the degree of renal development (Liang, Ioffe & Sun 1998). There has been a striking development in diagnostic protocol of FMRU within the last seven years (Vegar-Zubovic, Kristic & Lincender 2011). Urological pathologies can be noticeably detected and differentiated by the application of FMRU (Vegar-Zubovic, Kristic & Lincender 2011), which is a non invasive and feasible method in diagnosis of dilated urinary system, in both neonates and fetuses. Even in cases with obvious communication between genitourinary and gastrointestinal tracts; in which the diagnosis by ultrasonography is challenging, FMRU can be used as a practical method with shorter scanning time. Most of the urological abnormalities such as ureteropelvic junction (UPJ) stenosis, posterior urethral valve (PUV) or mega ureter can be sensitively detected by the application of FMRU. This meticulous diagnostic tool can play a crucial role in fetuses faced with complex anomalies. In addition, some of the coexisting anomalies can´t be detected in ultrasonography examination because of late referral (Sahinoglu et al. 2004). For these reasons, FMRU is regarded as a valuable tool in specification of urinary tract disorders. Although most of malformations are diagnosed in the last trimester of pregnancy (Gupta et al. 2010), the critical period for diagnosing the abnormalities and evaluating the possible need for termination of pregnancy is before 24 weeks of gestation.
In conclusion, we prenatally diagnosed almost all the specific details of CDS by the application of FMRU. Since this congenital malformation is accompanied with several complications that will result in neonatal death in most of the cases, its accurate prenatal diagnosis, its differentiation from other conditions with better prognosis can be crucial to the families. FMRU can be a finer diagnostic tool to characterize the defects identified by ultrasound in pregnant women confronted with possible CDS fetuses. However, more awareness of this technique would be of benefit in confirming cases with prenatal assumed diagnosis of CDS.
References
Achiron, R., Frydman, M., Lipitz, S. & Zalel, Y. (2000). “Urorectal Septum Malformation Sequence: Prenatal Sonographic Diagnosis in Two Sets of Discordant Twins,” Ultrasound in Obstetrics and Gynecology, vol. 16, no. 6, pp. 571-4.
Publisher – Google Scholar
Cerwinka, W. H., Grattan-Smith, J. D. & Kirsch, A. J. (2008). “Magnetic Resonance Urography in Pediatric Urology,”Journal of Pediatric Urology, vol. 4, no. 1, pp. 74-83.
Publisher – Google Scholar
Gupta, P., Kumar, S., Sharma, R. & Gadodia, A. (2010). “Case Report: Antenatal MRI Diagnosis of Cloacal Dysgenesis Syndrome,” The Indian Journal of Radiology & Imaging, vol. 20, no. 2, p. 143.
Publisher – Google Scholar
Liang, X., Ioffe, O. B. & Sun, C.-C., J. (1998). “Cloacal Dysgenesis Sequence: Observations in Four Patients Including Three Fetuses of Second Trimester Gestation,” Pediatric and Developmental Pathology, vol. 1, no. 4, pp. 281-8.
Publisher – Google Scholar
Pauli, R. M. (1994). “Lower Mesodermal Defects: A Common Cause of Fetal and Early Neonatal Death,” American journal of medical genetics, vol. 50, no. 2, pp. 154-72.
Publisher – Google Scholar
Sahinoglu, Z., Mulayim, B., Ozden, S., Etker, S., Celayir, A., Ozkan, F. & Bilgic, R. (2004). “The Prenatal Diagnosis of Cloacal Dysgenesis Sequence in Six Cases: Can the Termination of Pregnancy Always be the First Choice?,” Prenatal diagnosis, vol. 24, no. 1, pp. 10-6.
Publisher – Google Scholar
Vegar-Zubovic, S., Kristic, S. & Lincender, L. (2011). “Magnetic Resonance Urography in Children-When and Why?,”Radiology and Oncology, vol. 45, no. 3, pp. 174-9.
Publisher – Google Scholar
Warne, S., Chitty, L. S. & Wilcox, D. T. (2002). “Prenatal Diagnosis of Cloacal Anomalies,” BJU international, vol. 89, no. 1, pp. 78-81.
Publisher – Google Scholar