Discussion
Japanese Encephalitis which is caused by infection with the JE virus belongs to the mosquito-borne flavivirus group. Culex mosquito is the main vector while Pigs and some birds like herons and sparrows are the natural hosts. Most of the infected patient has subclinical infections or flu like symptoms. When fulminant acute neurological involvement occurs. Seizures and paralysis are seen and the condition carries a high mortality rate of 10% to 40% as mentioned by Matsui M. In up to 80% of those who survive there may be residual neurological findings. The definitive serologic diagnosis of JE is based on antibody detection in serum and CSF by immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay test.
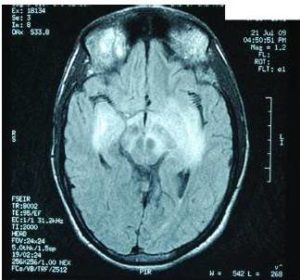
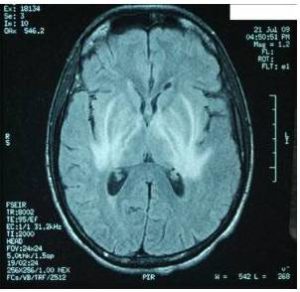
Characteristic MRI finding of JE is bilateral T2 thalamic yperintensity as said by Singh P and Ohtaki E as seen in Fig 2. Other common sites of involvement include substantia nigra, red nucleus and pons as seen in Fig 1. Hippocampus, cerebral cortex, and cerebellum are other common sites as mentioned in study by Ohtaki E , Abe T and Kumar S. Unilateral involvement has also been reported but is less common. Subcortical white matter involvement is also reported in some patients, but this has always been in combination with the more characteristic gray matter lesions. Some lesions, especially those in the thalamus, may be haemorrhagic. Enhancement is not usually observed, indicating only minor blood-brain barrier deficit. Abe et al discovered autoantibodies to myelin basic protein and neurofilaments in some patients with JE and raised the possibility of a superimposed immunologic component to this disease that may selectively involve certain portions of the central nervous system.
Uremic encephalopathy also involves nervous system as small hyperintensities in subcortical parieto-occipital region and in deep brain parenchyma, which regresses as the patient condition, improves as said in research by Schmidt M and Okada J. In above case the patient did not have any prior history of renal dysfunction. Coexistent of both the condition in same patient is rare with mark signal changes in MRI and possibility of autoimmune mechanism against renal tissue.
Conclusions
JE leads to typical MRI findings in brain and it is very helpful as an additive tool in its diagnosis. Uremic encephalopathy also involves nervous system and its MRI findings are almost same. In such situation other pathological examinations result also has to be considered.
References
Abe, T., Kojima, K., Shoji, H. et al. (1998). “Japanese Encephalitis,” J Magn Reson Imaging 1998; 8:755-761.
Publisher – Google Scholar
Kumar, S., Misra, U. K., Kalita, J. et al. (1997). “MRI in Japanese Encephalitis,” Neuroradiology 1997;39: 180-184.
Publisher – Google Scholar
Matsui, M., Kawano, H., Matsukura, M., Otani, Y. & Miike, T. (2002). “Acute Transverse Myelitis after Japanese B Encephalitis Vaccination in a 4-Year-Old Girl,” Brain Dev. 2002 Apr; 24(3):187-9
Publisher – Google Scholar
Ohtaki, E., Murakami, Y., Komori, H., Yamashita, Y. & Matsuishi, T. (1992). “Acute Disseminated Encephalomyelitis after Japanese B Encephalitis Vaccination,” Pediatr Neurol. 1992 Mar-Apr; 8 (2):137-9
Publisher – Google Scholar
Okada, J., Yoshikawa, K., Matsuo, H., Kanno, K. & Oouchi, M. (1991). “Reversible MRI and CT Findings in Uremic Encephalopathy,” Neuroradiology Volume 33, Number 6 / November, 1991524-526
Publisher – Google Scholar
Schmidt, M., Sitter, T., Lederer, S. R., Held, E. & Schiffl, H. (2001). “Reversible MRI Changes in a Patient with Uremic Encephalopathy,” J Nephrol. 2001 Sep-Oct; 14(5):424-7
Publisher – Google Scholar
Singh, P., Kalra, N., Ratho, R. K. et al. (2001). “Coexistent Neurocysticercosis and Japanese B Encephalitis: MR Imaging Correlation,” American Journal of Neuroradiology 22:1131-1136 (6 2001)
Publisher – Google Scholar